what are the possible reduction products in the experiment? select all that apply. select one or more:
Answers
The order to provide you with the correct possible reduction products in the experiment, I would need more information about the specific experiment and the chemicals involved. Once you provide those details, identify the possible reduction products. The atom that loses electrons is oxidized, and the atom that gains electrons is reduced.
To understand electron-transfer reactions like the one between zinc metal and hydrogen ions, chemists separate them into two parts one part focuses on the loss of electrons, and one part focuses on the gain of electrons. The loss of electrons is called oxidation. The gain of electrons is called reduction. Because any loss of electrons by one substance must be accompanied by a gain in electrons by something else, oxidation and reduction always occur together. As such, electron-transfer reactions are also called oxidation-reduction reactions, or simply redox reactions. The atom that loses electrons is oxidized, and the atom that gains electrons is reduced. Also, because we can think of the species being oxidized as causing the reduction, the species being oxidized is called the reducing agent, and the species being reduced is called the oxidizing agent.
learn more about reduction here.
https://brainly.com/question/15265712
#SPJ11
Related Questions
Predict the bond angles for each of the following labeled bonds.
The answer choices are 120, 111.4, and 124.3
See the picture attached.
Note: Here is a link to the answer in case any one else is struggling and needs an explanation: https://brainly.com/question/13899295

Answers
The bond angles a and b are 120° respectively. The bond angle c is 111.4° .while the bond angle d is 120°. The bond angles e and f are 120° respectively.
In the carbonate ion, all the bond angles and bond lengths are equal hence three equivalent resonance structures can be drawn for the ion. All the bond angles, ( a and b) in carbonate ion all have bond angle of 120°.
The bond angle marked c in OCCl2 has a bond angle 111.4°, the bond angle marked d in the compound has the bond angle, 120°.
There are three bond angles present in the nitrate (NO3-) ion. Three resonance structures contribute to this bond. Based on these structures, the bond angles e and f in the molecule is 120°.
Learn more: https://brainly.com/question/20339399
Name three perfessinial areas in the u.s thaT use meTric system
Answers
The three professional areas that uses metric system are the scientific fields, medical fields, or technical fields.
What is metric system?The metric system is a measurement method that utilizes the meter, liter, as well as gram as base units for length for distance, capacity for volume, and weight for mass.
The primary factors for the United States' failure to embrace the metric system are simply cost and time.
When the country's Industrial Revolution began, expensive manufacturing plants was becoming a major source of American jobs and basic goods.
Thus, the scientific, medical, and technical fields are the three professional fields that use the metric system.
For more details regarding metric system, visit:
https://brainly.com/question/25966695
#SPJ1
Please help How many moles of a gas sample are in 5.0 L container at 215 K and 342 kPa(The gas constant is 8.31 L kPa/mol K) Round your answer to one decimal place and enter the number only with no units.

Answers
Answer
1.0 mol
Explanation
Given:
Volume, V = 5.0 L
Temperature, T = 215 K
Pressure, P = 342 kPa
The gas constant, R = 8.31 L kPa/mol K
What to find:
The number of moles of the gas sample.
Step-step-solution:
The number of moles of the gas can be determine using the ideal gas equation formula:
\(PV=nRT\)Put the given values into the formula and calculate for n:
\(\begin{gathered} 342\times5.0=n\times8.31\times215 \\ 1710=1786.65n \\ \text{Divide both sides by 1786.65} \\ \frac{1710}{1786.65}=\frac{1786.65n}{1786.65} \\ n=0.9571\text{ mol} \\ To\text{ one decimal place,} \\ n=1.0\text{ mol} \end{gathered}\)The number of moles of the gas sample is 1.0 mol.
How does water get up to the atmosphere, and how does it get back down to Earth’s surface?
Answers
Answer: water goes into atmosphere through evaporation and get back down on earth surface by precipitation.
Explanation: Evaporation is the process where the water gets evaporated to the atmosphere where the liquid state of the water is changed into the vapor state . Then the vapor condenses into the clouds till the clouds get filled and the water falls as precipitation to the ground or earth. This whole process is called the water cycle.
Briefly answer the following questions, including reasoning and calculations where appropriate: (a) Explain in your own words why direct expansion systems require the vapour exiting the evaporator to be superheated. (8 Marks) (b) Describe the difference between a forced draft evaporator and an induced draft evaporator, and describe why (and in what type of system) a forced draft evaporator is often preferred over an induced draft evaporator. (6 Marks) (c) Determine the R-number of each of the following refrigerants, and hence classify them (ie chlorofluorocarbon, hydrocarbon etc): (i) CClF 2
CF 3
(3 Marks) (ii) Tetrafluoroethane (3 Marks) (iii) H 2
O (3 Marks) (d) Briefly describe the role of hydrogen gas in an absorption refrigeration system (NH 3
/H 2
O/H 2
). In a system where the evaporating temperature is −2.0 ∘
C, with a design condensing temperature of 38.0 ∘
C, estimate the partial pressure of hydrogen in the evaporator.
Answers
Direct expansion systems require the vapour exiting the evaporator to be superheated to avoid liquid slugging, to improve the effectiveness of the evaporator and to maintain the stability of the compressor. (B) Forced draft and induced draft evaporators differ in the way air is introduced into them. (C) CClF2CF3 (also known as R12) is a chlorofluorocarbon refrigerant. (ii) Tetrafluoroethane (also known as R134a) is a hydrofluorocarbon refrigerant and H2O is not classified as a refrigerant. (D) The partial pressure of hydrogen in the evaporator is 1.6 mmHg.
(a) Direct expansion systems are those in which the refrigerant in the evaporator evaporates directly into the space to be cooled or frozen. The evaporator superheat is used to make sure that only vapor and no liquid is carried over into the suction line and compressor. Superheating is required for the following reasons :
To avoid liquid slugging : Liquid slugging in the compressor's suction line can be caused by a lack of superheat, which can result in compressor damage. To improve the effectiveness of the evaporator : Superheating increases the evaporator's efficiency by allowing it to absorb more heat. To maintain the stability of the compressor : The compressor is protected from liquid by the correct use of superheat, which ensures that only vapor is returned to the compressor.(b) Forced draft and induced draft evaporators differ in the way air is introduced into them. In an induced draft evaporator, a fan or blower is positioned at the top of the evaporator, and air is drawn through the evaporator from the top. In a forced draft evaporator, air is propelled through the evaporator by a fan or blower that is located at the bottom of the evaporator. Forced draft evaporators are frequently used in direct expansion systems because they allow for better control of the air temperature. Because the air is directed upward through the evaporator and out of the top, an induced draft evaporator is less effective at keeping the air at a uniform temperature throughout the evaporator.
(c) (i) CClF2CF3 (also known as R12) is a chlorofluorocarbon refrigerant.
(ii) Tetrafluoroethane (also known as R134a) is a hydrofluorocarbon refrigerant.
(iii) H2O is not classified as a refrigerant.
(d) The function of hydrogen gas in an absorption refrigeration system (NH3/H2O/H2) is to increase the heat of reaction between ammonia and water.
The pressure of hydrogen gas in the evaporator of an absorption refrigeration system can be determined using the formula, Pa/Pb = (Ta/Tb)^(deltaS/R),
where Pa = partial pressure of hydrogen in the evaporator, Ta = evaporating temperature, Tb = condensing temperature, Pb = partial pressure of hydrogen in the absorber, deltaS = entropy change between the absorber and evaporator, R = gas constant.
Substituting the given values, Ta = −2.0 ∘C = 271 K ; Tb = 38.0 ∘C = 311 K ; Pb = atmospheric pressure = 1 atm ;
deltaS = 4.7 kJ/kg K ; R = 8.314 kJ/mol K
we get, Pa/1 atm = (271/311)^(4.7/8.314)
Pa = 0.021 atm or 1.6 mmHg
Therefore, the partial pressure of hydrogen in the evaporator is 1.6 mmHg.
Thus, Direct expansion systems require the vapour exiting the evaporator to be superheated to avoid liquid slugging, o improve the effectiveness of the evaporator and to maintain the stability of the compressor. (B) Forced draft and induced draft evaporators differ in the way air is introduced into them. (C) CClF2CF3 (also known as R12) is a chlorofluorocarbon refrigerant. (ii) Tetrafluoroethane (also known as R134a) is a hydrofluorocarbon refrigerant and H2O is not classified as a refrigerant. (D) The partial pressure of hydrogen in the evaporator is 1.6 mmHg.
To learn more about chlorofluorocarbons :
https://brainly.com/question/18414838
#SPJ11
Which of the following shows three elements in order of increasing conductivity?
Mn, Ge, o
Mn, O, Ge
0, Ge, Mn
Ge, O, Mn
Answers
Answer:
its c
Explanation:
its c
PLEASE HELP WITH THESE THREE (30 POINTS)
1) How is the number of neutrons in the nucleus of an atom calculated?
A. Add the number of elections- and protons+ together
B. Subtract the number of elections- from protons+
C. Subtract the number of protons+ from the atomic mass number
D. Add the atomic mass number to the number of elections-
2) Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), and Francium (Fr) are in the same group on the periodic table. Why? *
A. They have the same size.
B. They react with each other.
C. They have similar properties.
D. They have the same number of protons
3) Which of the following elements would be a transition metal?
A. Ca
B. Au
C. Rb
D. Fr
Answers
2. C
3. A
i had the test with the same exact questions
please help!!!!
A certain reaction is endothermic in the forward direction. The reaction has less moles of gas on the product side. Which of the following stresses would increase the yield of the product (shift right)?
a) increase the temperature
b) decrease the temperature
c) increase the volume
d) decrease the reaction concentration
Answers
Answer:
The correct answer is A, "increase the temperature."
Explanation:
By increasing the temperature, it would shift the reaction to the right. All of the other options would either cause the reaction to shift left, or are simply invalid. I hope this helps!
Increase in temperature will increase the yield of the product and cause a shift to the right.
What is an Endothermic reaction?This is the type of reaction in which reactants absorb heat energy from the surroundings to form products.
This depicts that an increase in temperature will increase the yield of products thereby making option A the most appropriate choice.
Read more about Endothermic reaction here https://brainly.com/question/1447631
Which of the following reactions
is BALANCED and shows
COMPLETE combustion?
A. CsH12 + 8025CO2 + 6H₂O
B. 2CsH12 + 110210CO + 12H₂O
C. CsH12 + 8026CO2 + 5H₂O
D. 2CsH12 + 1102 12CO + 10H₂O
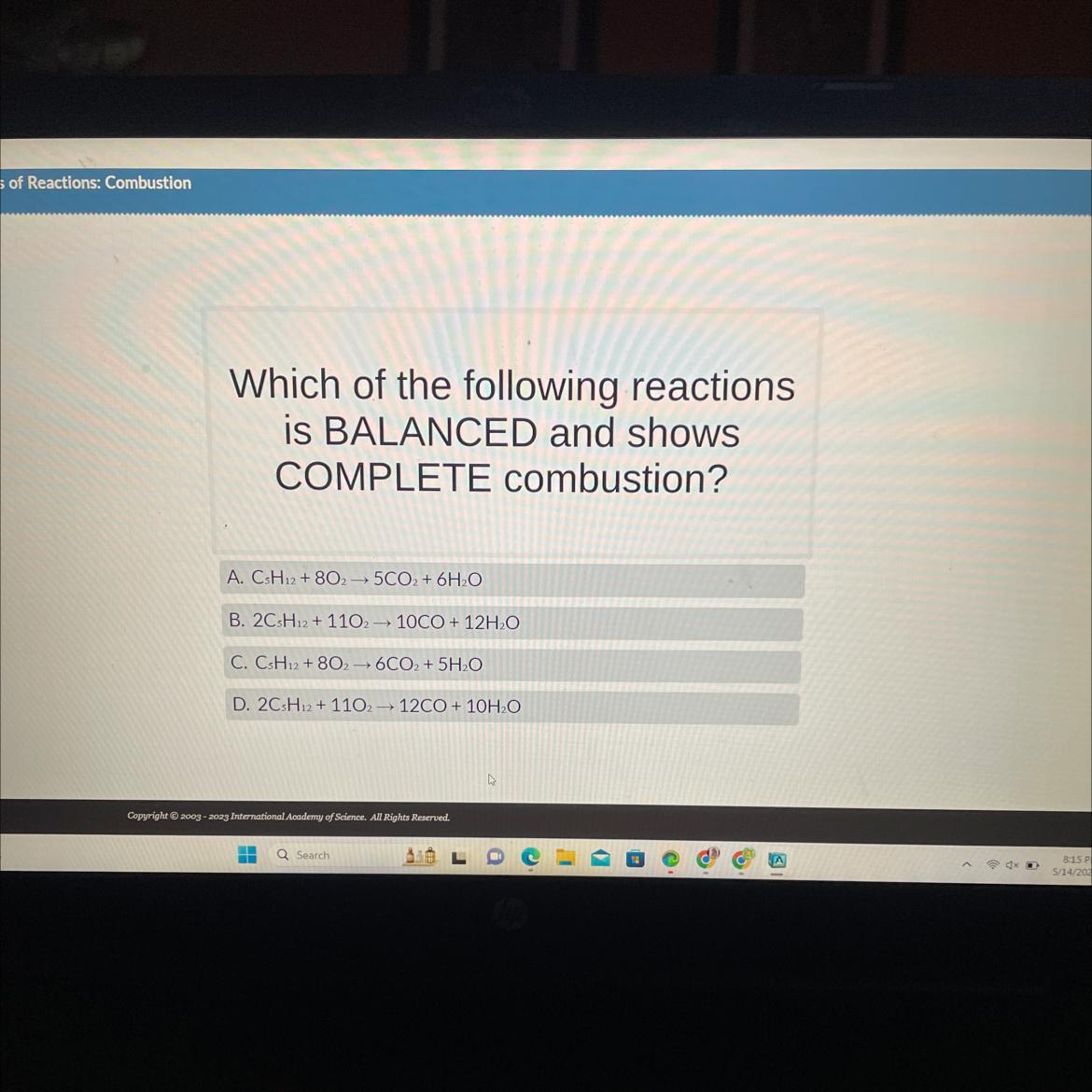
Answers
From the analysis, we can see that option D is balanced equations and shows complete combustion. Hence, the correct option is D.
To determine which of the given reactions is balanced and shows complete combustion, we need to check if the number of atoms of each element is balanced on both sides of the equation. Additionally, complete combustion occurs when the reactant hydrocarbon is completely converted to carbon dioxide (CO2) and water (H2O).
Let's analyze each reaction:
A. CsH12 + 8025CO2 + 6H₂O
In this reaction, there are 25 carbon atoms on the product side but only 1 carbon atom on the reactant side. It is unbalanced and does not represent complete combustion.
B. 2CsH12 + 110210CO + 12H₂O
This reaction has 210 carbon atoms on the product side but only 4 carbon atoms on the reactant side. It is unbalanced and does not represent complete combustion.
C. CsH12 + 8026CO2 + 5H₂O
In this reaction, there are 26 carbon atoms on the product side but only 1 carbon atom on the reactant side. It is unbalanced and does not represent complete combustion.
D. 2CsH12 + 1102 12CO + 10H₂O
This reaction has 12 carbon atoms on both sides of the equation, which means it is balanced in terms of carbon. It also represents complete combustion as it produces carbon dioxide and water as the only products. Therefore, the correct option is D.
The balanced and complete combustion reaction is:
2CsH12 + 1102 → 12CO2 + 10H₂O
For more question on complete combustion click on
https://brainly.com/question/17419608
#SPJ11
8.45 cm - 3.55 cm = ?
Answers
Answer:
4.9cm or 4.90cm
Explanation:
Use column method and align the numbers. Make sure you add the decimal point before you write the answer, otherwise the value would be wrong.
Answer:
4.90
Explanation:
8.45.
-3.55
minus those two and you'll get the sum!
Question 3 (1 point)
Electrochemical processes involve redox reactions because electrons are transferred.
O True
O False
Question 4 (1 point)
The activity series of metals helps determine the result of chemical reactions.
O True
O False
Answers
An electrochemical process involves any process that has to do with the transfer of elecrons.
What is an elecrochemical process?An electrochemical process involves any process that has to do with the transfer of elecrons. These process include redo reactionand electron transport chains.
It is a true statement that the actiivity series helps to detect the products in a reaction because a metal that is lower in the series is always displaced by a metal that is higher in the series.
Learn more about electrochemical processess: https://brainly.com/question/12034258
*Fill in the blank about the periodic table*
Atomic number equals the number of ______ or ______
Atomic mass equals the number of _____ + ______
Answers
Atomic number equals the number of protons or electrons. Atomic mass equals the number of protons and neutrons
a bomb calorimetry measurement indicates that a single potato chip has a heat content os 20,000j. what is on the label of a bag of potato chips that contains 20 chips?
Answers
The label of a bag of potato chips that contains 20 chips using calorimetry will be 400,000J.
What is calorimetry?Calorimetry is the science of measuring the heat absorbed or evolved during the course of a chemical reaction or change of state.
A bomb calorimeter is a type of constant-volume calorimeter in which material is burned to measure its heat content.
According to this question, a bomb calorimetry measurement indicates that a single potato chip has a heat content of 20,000J. This amount is specifically for only one chip, hence, the amount of multiple chips can easily be calculated as follows:
Heat energy of 20 chips = 20,000J × 20 chips
Heat energy of 20 chips = 400,000J
Therefore, 400,000J of energy will be on the label bag.
Learn more about calorimetry at: https://brainly.com/question/24245395
#SPJ1
A 5 cm3 piece of aluminum has a higher density than a 10cm3 of aluminum. True or false
Answers
Density stays the same, false
in the energy and specific heat lab, you measure the temperature change of water to study the specific heat of a metal. what statement explains the relationship between the water and the metal you are studying? select one: the heat lost by the metal plus the heat gained by the water equals 100. the initial temperature of the metal equals the initial temperature of the water. the temperature change of the metal is equal to the temperature change of the water. the heat lost by the metal is equal to the heat gained by the water.
Answers
in the energy and specific heat lab, you measure the temperature change of water to study the specific heat of a metal. the statement which explains The relationship between The water and The metal is "The heat lost by the metal is equal to the heat gained by the water".
What is specific heat?The heat required to raise a mole of material's heat content by precisely one degree Celsius is known as the heat capacity, or Cp.
The following method can be used to calculate specific heat values: Heat always transfers from the warmer to the colder material until both materials reach the same temperature when two materials, each at an initial different temperature, are placed in contact with one another. The heat gained by the initially colder material must equal the heat lost by the initially warmer material, according to the law of conservation of energy.
To determine particular heat values, apply the procedure described below: When two materials, each at an initial different temperature, are placed in contact, heat always transfers from the warmer to the cooler material until both materials reach the same temperature. According to the law of conservation of energy, the heat gained by the initially colder substance must equal the heat lost by the initially warmer substance.
mathematically, Q = mC∆T
Where
Q = quantity of heat absorbed by a body
m = mass of the body
∆t = Rise in temperature
C = Specific heat capacity of a substance depends on the nature of the material of the substance.
S.I unit of specific heat is J kg-1 K-1.
Learn more about specific heat here :
brainly.com/question/27991746
#SPJ4
plss help . i have no idea

Answers
Answer:
Protons and Neutrons have approximately the same mass.
Equal numbers of protons and electrons make an atom neutral.
Protons have a positive charge
Explanation:
Protons and Neutrons have approximately the same mass. - both are 1 amu
Equal numbers of protons and electrons make an atom neutral. - protons are +1 and electrons are -1, so they cancel each other if there are equal amounts.
Protons have a positive charge - this is true.
What law states that matter cannot be created nor
destroyed, even in a chemical reaction?
A
Newton's Laws of Motion
B
Law of Conservation of Mass
Answers
Answer:
I believe it would be Law of conservation of mass (sorry if I'm incorrect)
giải gấp giúp em câu này với ạ:
a) Hoàn thành các phan ứng sau dưới dạng phương trình phân tử và phương trình ion thu gọn:
- CaCO3 + HCl =>
- Fe(SO4)3 + NaOH =>
- KCl + ? => KNO3 + ?
- Na2S + H2SO4 =>
b) Viết PTPU của các phương trình ion thu gọn sau, xác định chất nào là acid, chất nào là bazo:
- H(+) + OH(-) => H2O
- OH(-) + HCO3(-) => CO3(2-) + H20
Answers
Explanation:
what do you have written I can't understand one word right a properly
to which third-period element do these ionization values belong? spell out the full name of the element.
Answers
The ionization values belong to the element magnesium (Mg). Ionization values help us determine the amount of energy that will be required to remove one or more electrons from an atom. The third period is where we can locate the element that has these ionization values.
These ionization values are listed in the table given below:Element: Mg (Magnesium)First ionization energy: 738 kJ/molSecond ionization energy: 1450 kJ/molThird ionization energy: 7732.7 kJ/mol
For a neutral atom, the first ionization energy (IE1) is the amount of energy required to remove an electron from the outermost shell. As we move from left to right in a period, the ionization energy increases. In the third period, Mg (magnesium) has first, second, and third ionization energies of 738 kJ/mol, 1450 kJ/mol, and 7732.7 kJ/mol, respectively. Magnesium is a chemical element that has an atomic number of 12. It has two valence electrons and is located in group 2 of the periodic table. Magnesium has a melting point of 1,202°F (650°C) and a boiling point of 1,994°F (1,090°C). It is a silver-white metal that is widely used in the manufacture of alloys and other industrial applications.
learn more about ionization energy
https://brainly.com/question/20658080
#SPJ11
tell me everything you know about thermal energy im in school right now and i have to write an essay :)
Answers
Answer:
thermal energy is heat energy it is used for man kitchen appliances and also in nature from the sun for photosynthesys
Explanation:
all amino acids share a common structure, a central (alpha) carbon with four groups attached. which of these is not one of the four groups attached to the alpha-carbon
Answers
Among the options given, the hydrogen atom (H) is not one of the four groups attached to the alpha-carbon.
In an amino acid, the central (alpha) carbon is indeed bonded to four groups. These four groups are:
Amino group (-NH₂): This group consists of a nitrogen atom bonded to two hydrogen atoms. It is called the amino group and gives amino acids their name.
Carboxyl group (-COOH): This group consists of a carbon atom double-bonded to an oxygen atom and single-bonded to a hydroxyl group (-OH). It is called the carboxyl group and is responsible for the acidic properties of amino acids.
Hydrogen atom (H): A single hydrogen atom is also attached to the central carbon.
Side chain (R group): The fourth group attached to the alpha-carbon is the side chain, also known as the R group. The side chain varies among different amino acids and gives each amino acid its unique properties.
Therefore, among the options given, the hydrogen atom (H) is not one of the four groups attached to the alpha-carbon.
The completed question is given as,
All amino acids share a common structure, a central (alpha) carbon with four groups attached. Which of these is NOT one of the four groups attached to the alpha- carbon?
an R group
an ammonium group (-NH³⁺)
a carboxylate group (-COO)
a hydrogen atom
Learn more about amino acid from the link given below.
https://brainly.com/question/31442968
#SPJ4
Which of these describes why a stent is used?
O A. To open an artery near the heart
O B. To repair a weak heart valve
O C. To increase the biocompatibility of a heart transplant
O D. To enhance the durability of a pacemaker
SUBMIT
Answers
Answer:the correct answer is A.To open an artery near the heart
Explanation:I just took the test :)
A stent is used to open an artery near the heart and the correct option is option A.
A stent is commonly used in medical procedures to open up narrowed or blocked arteries near the heart. It is a small, mesh-like tube typically made of metal or synthetic materials. The stent is inserted into the affected artery and expands, effectively widening the artery and improving blood flow. This procedure is known as coronary angioplasty or stenting.
The narrowing or blockage of arteries can occur due to atherosclerosis, a condition in which plaque builds up on the artery walls. This can lead to reduced blood flow and potentially result in chest pain (angina) or even a heart attack. By placing a stent, the artery is opened up, allowing for improved blood flow to the heart muscle.
Thus, the ideal selection is option A.
Learn more about Stent, here:
https://brainly.com/question/30477421
#SPJ6
I need help for this really the image is attached

Answers
The net ionic equations of the reactions are;
3Mn^3+(aq) + 4PO4^3-(aq) ----> Mn3(PO4)4(s)
Pd^2+(aq) + S^2-(aq) ----> PdS(s)
What is the net ionic equation?A net ionic equation is a chemical equation that shows only the species that participate in a reaction and contribute to the formation of a product or the consumption of a reactant.
It excludes any spectator ions that do not undergo a chemical change. In other words, it shows the actual chemical species that are involved in the reaction.
Learn more about net ionic equation:https://brainly.com/question/29299745
#SPJ1
A gas mixture is made from 15.6 g of bromine gas and 13.8 g of chlorine gas. The total pressure of the mixture is 0.555 atm. What is the partial pressure of the bromine gas?
Answers
Answer:
the partial pressure of bromine gas is 0.186 atm
Explanation:
Which of the processes below is a way scientists can accelerate the process
of traditional crossbreeding through inserting beneficial traits in the DNA of a
plant?
Answers
Answer:
Genetic Engineering
Explanation:
AP3X
the following experiment was carried out using a newly synthesized chlorofluorocarbon. exactly 50 ml of the gas effused through a porous barrier in 157 s. the same volume of argon effused in 76 s under the same conditions. which compound is the chlorofluorocarbon?
Answers
The molar mass corresponds to the chlorofluorocarbon CF3Cl (Freon-11), which has a molar mass of 137.37 g/mol. Therefore, the chlorofluorocarbon in the experiment is CF3Cl.
The rate of effusion of a gas through a porous barrier is inversely proportional to the square root of its molar mass. Therefore, we can use the rate of effusion to determine the relative molar mass of the two gases and identify which one is a chlorofluorocarbon.
The rate of effusion can be calculated using Graham's law:
Rate of effusion = Volume of gas / Time taken to effuse
For the chlorofluorocarbon, the rate of effusion is:
Rate of effusion (CFC) = 50 mL / 157 s = 0.3185 mL/s
For argon, the rate of effusion is:
Rate of effusion (Ar) = 50 mL / 76 s = 0.6579 mL/s
Using Graham's law, we can set up the following equation:
Rate of effusion (CFC) / Rate of effusion (Ar) = sqrt(Molar mass (Ar) / Molar mass (CFC))
Solving for the ratio of molar masses:
Molar mass (Ar) / Molar mass (CFC) = (Rate of effusion (Ar) / Rate of effusion (CFC))^2
Molar mass (Ar) / Molar mass (CFC) = (0.6579 mL/s / 0.3185 mL/s)^2
Molar mass (Ar) / Molar mass (CFC) = 4.294
Molar mass (CFC) = Molar mass (Ar) / 4.294
The molar mass of argon is 39.95 g/mol. Therefore, the molar mass of chlorofluorocarbon is:
Molar mass (CFC) = 39.95 g/mol / 4.294 = 9.30 g/mol
To learn more about molar mass
https://brainly.com/question/13152455
#SPJ4
which of the following is true of a hydrocarbon? group of answer choices it consists of carbon and hydrogen atoms. it can form a ring structure. it is a good fuel for combustion reactions. it can contain double or triple bonds. all of these
Answers
The following is true of a hydrocarbon is e. All of these
Hydrocarbon compounds are the simplest carbon compounds composed of carbon and hydrogen atoms that can form ring structures, are used as fuel for combustion reactions, and contain double or triple bonds.
The common characteristics of hydrocarbons are that they produce steam, carbon dioxide, and heat during combustion, and oxygen is required for the combustion reactions to occur. This compound is used as a fuel source. In everyday life we encounter many carbonate compounds, such as kerosene, gasoline, natural gas, and plastics. Other types of hydrocarbons such as propane and butane are used in Liquified Petroleum Gas and some materials for making medicine and clothing.
Learn more about hydrogen at:
https://brainly.com/question/29765115
#SPJ11
Summarize how the structure of organic compounds allows them to function as pigments in 2 – 3 sentences
Answers
The structure of organic compounds allows them to function as pigments through chromophore and acid or basic groups.
What is a Pigment?This is defined as a colored substance which is completely or nearly insoluble in water.
The structure of organic compounds allows them to function as pigments include chromophore and the acid or basic groups such as OH, SO3H, etc.
Read more about Pigment here vhttps://brainly.com/question/1056549
#SPJ1
What is the molar mass of sulfur (S)?
A. 32.06 g/mol
OB. 16 g
O c. 16 g/mol
D. 32.06 g
Answers
Answer:
A. 32.06 g/mol
Explanation:
The molar mass units are always g/mol
its 32.06 g/mol bro its letter A
What will the pH of 1.50 L of pure water water be if 2.0 mL of 4.0 M HCl is added? By how much has the pH changed? What will the pH of the solution in part b be if 2.0 mL of 4.0 M HCl is added? By how much has the pH changed?
Answers
Answer:
Part A
pH ≈ 2.273
Part B
ΔpH ≈ -4.726
Part C
pH ≈ 1.973
Part D
ΔpH ≈ -0.301
Explanation:
Part A
The pH of a solution is given by the negative concentration of hydrogen ions in the solution
2.0 mL = 0.002 L
The number of moles of HCl in 2.0 mL of 4.0 M HCl is given as follows;
1 Liter of 4.0 M HCl contains 4.0 moles of HCl
2.0 mL = 0.002 L 4.0 M HCl contains 0.002 L/(1 L) × 4.0 M = 0.008 moles of HCl
The concentration of 0.008 moles in 1.50 L is given as follows;
Concentration = The number of moles/(The volume in liters)
∴ The concentration of 0.008 moles in 1.50 L, C = 0.008 moles/(1.5 L + 0.002 L)
∴ The concentration of 0.008 moles in 1.50 L, C ≈ 0.00533 moles/liter = 0.00533 M HCl
Given that HCl is a strong acid, we have that HCl dissociates completely to give equal number of H⁺ and Cl⁻ ions;
The number of moles of [H⁺] in the solution = 0.00533 moles
The pH of the solution = -log[H⁺]
∴ pH = -log[5.33 × 10⁻³] ≈ 2.273
The pH of the 1.5 L of pure water will be approximately 2.273
Part B
The pH of the pure water has changed from neutral (pH = 7) tp pH = 2.273
The change in pH is ΔpH = 2.274 - 7 = -4.726
ΔpH ≈ -4.726
Part C
When 2.0 mL of the 4.0 M HCl is added, the solution above, we have;
C = (0.008 + 0.008)/(1.5 + 0.002 + 0.002) ≈ 1.06383 × 10⁻²
The concentration of the solution becomes, C ≈ 1.06383 × 10⁻² mole/liter
The pH becomes, pH = -log(1.06383 × 10⁻²) ≈ 1.973
Part D
The amount by which the pH has changed, ΔpH ≈ 1.973 - 2.274 = -0.301.