What answer did you calculate for the ph after the reaction of 100 ml of 0. 1 m nh3 and 110 ml of 0. 1 m hno3?.
Answers
The pH after the reaction of 100 ml of 0.1 M NH3 and 110 ml of 0.1 M HNO3 is 4.74.Explanation:The reaction of NH3 and HNO3 is a type of acid-base neutralization reaction in which NH3 acts as a base and HNO3 acts as an acid.
The balanced chemical equation for the reaction is:NH3 + HNO3 → NH4NO3The reaction equation indicates that 1 mole of NH3 reacts with 1 mole of HNO3 to produce 1 mole of NH4NO3. The reaction consumes H+ ions from the HNO3 and produces NH4+ ions from the NH3. The concentration of H+ ions decreases and the concentration of OH- ions increases, leading to an increase in pH. Since the NH3 is present in excess, it is the limiting reactant. Hence, the amount of NH3 remaining unreacted is equal to the initial amount of NH3 which is 0.1 mol. The amount of HNO3 reacted is equal to the initial amount of HNO3 minus the amount of NH3 reacted, which is 0.1 - 0.1 = 0 mol. The amount of NH4NO3 produced is equal to the amount of NH3 reacted, which is 0.1 mol. Using the stoichiometry of the reaction, we can calculate the molarity of NH4NO3 produced, which is:Molarity = moles / volume Molarity = 0.1 mol / (100 ml + 110 ml)Molarity = 0.1 mol / 210 mlMolarity = 0.000476 MThe pH of a solution of NH4NO3 can be calculated using the following formula:pH = pKa + log([NH4+]/[NH3])The pKa of NH4+ is 9.24. The concentration of NH4+ and NH3 can be calculated using the molarity of NH4NO3 and the amount of NH3 remaining unreacted, which is:Molarity = moles / volumeMoles = molarity x volumeVolume = 100 ml = 0.1 L (since 1 ml = 0.001 L)Moles of NH4+ = 0.000476 M x 0.1 L = 0.0000476 molesMoles of NH3 = 0.1 mol - 0.0000476 moles = 0.0999524 moles[NH4+] = 0.0000476 moles / 0.210 L[NH3] = 0.0999524 moles / 0.210 L[NH4+]/[NH3] = (0.0000476 moles / 0.210 L) / (0.0999524 moles / 0.210 L)[NH4+]/[NH3] = 0.000226The pH can be calculated as:pH = 9.24 + log(0.000226)pH = 4.74Therefore, the pH after the reaction of 100 ml of 0.1 M NH3 and 110 ml of 0.1 M HNO3 is 4.74.
For more information on Molarity visit:
brainly.com/question/31545539
#SPJ11
Related Questions
Please actually help me.
Thank you!

Answers
Answer:
I say 20 is ur best option
If 12.3 mol HCl are produced in this reaction, how many grams of sodium sulfate are produced?

Answers
ANSWER
The mass of Na2SO4 is 874g
EXPLANATION
Given that;
The number of moles of HCl is 12.3 mol
Follow the steps below to find the mass of sodium sulfate produced
Step 1; Write a balanced equation for the reaction
\(\text{ 2NaCl + H}_2SO_4\text{ }\rightarrow\text{ 2HCl + Na}_2SO_4\)In the reaction above, 2 moles of NaCl react with 1 mole of H2SO4 to give 2 moles of HCl and 1 mole of Na2SO4
Let the number of moles of Na2SO4 be x
\(\begin{gathered} \text{ 2 moles HCl }\rightarrow\text{ 1 mole Na}_2SO_4 \\ \text{ 12.3 moles HCl }\rightarrow\text{ x moles Na}_2SO_4 \\ \text{ Cross multiply} \\ \text{ 2 moles HCl }\times\text{ x moles Na}_2SO_4\text{ }=\text{ 1 mole Na}_2SO_4\text{ }\times\text{ 12.3 mole HCl} \\ \text{ Isolate x} \\ \text{ }\times\text{ }=\text{ }\frac{1\text{ mole Na}_2SO_4\times12.3mol\cancel{HCl}}{2moles\cancel{HCl}} \\ \text{ } \\ \text{ x = }\frac{1\text{ }\times\text{ 12.3}}{2} \\ \text{ x = 6.15 moles} \end{gathered}\)The number of moles of Na2SO4 is 6.15 moles
Step 3; Find the mass of Na2SO4 using the below formula
\(\text{ mole = }\frac{\text{ mass}}{\text{ molar mass}}\)Recall, that the molar mass of Na2SO4 is 142.04 g/mol
\(\begin{gathered} \text{ mass = mole }\times\text{ molar mass} \\ \text{ mass = 6.15 }\times\text{ 142.04} \\ \text{ mass = 873.546} \\ \text{ mass = 874g Na}_2SO_4 \end{gathered}\)Therefore, the mass of Na2SO4 is 874g
C3.26 The diagram shows the arrangement of the outer electrons only in a molecule of ethanoic acid.
a, Name the different elements found in this
compound.
b What is the total number of atoms present in
this molecule?
C
Between which two atoms is there a double
covalent bond?
d
How many covalent bonds does each carbon
atom make?
e
Would you expect this compound to be a solid
or a liquid at room temperature? Give a reason
for your answer.
f,
Ethanoic acid will dissolve in methylbenzene.
Would you expect the solution to conduct
electricity? Give a reason for your answer.
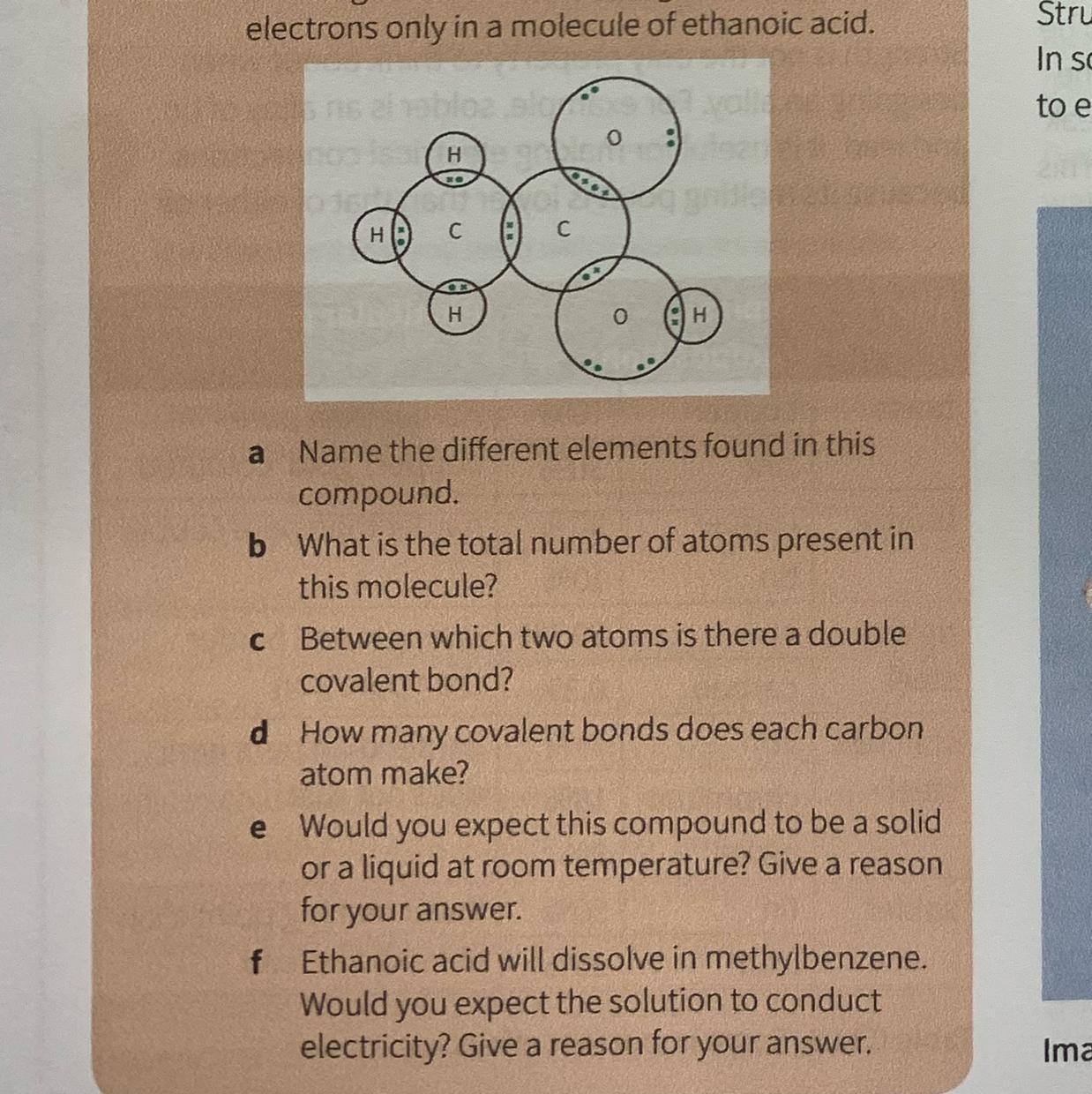
Answers
The diagram shows the arrangement of the outer electrons only in a molecule of ethanoic acid.
elements are = Carbon, Hydrogen and oxygentotal no. of atoms = 8double bond = between one carbon and oxygentotal covalent bond = 7liquid at room temperaturea) The different elements found in this compound is Carbon, Hydrogen and oxygen.
b) The total number of atoms present in this molecule is 8.
c) Between which two atoms is there a double covalent bond is in the between of one carbon atom and oxygen atom.
d) number of the covalent bonds does each carbon atom make is 7.
e) this compound to be a liquid at room temperature
Thus, The diagram shows the arrangement of the outer electrons only in a molecule of ethanoic acid.
elements are = Carbon, Hydrogen and oxygentotal no. of atoms = 8double bond = between one carbon and oxygentotal covalent bond = 7liquid at room temperatureTo learn more about covalent bond here
https://brainly.com/question/10777799
#SPJ1
what are possible constraints, or challenges, which would prevent you from getting the features you listed?
Answers
The three primary constraints that project managers should be familiar with are time, scope, and cost.
What are some examples of constraints?
Stretched resources.Operational mishaps.Low performance.Lack of clarity.Scope creep.High costs.Time crunch.What are the 4 common types of constraints facing services?
The most common types of constraints facing service businesses are Time, Labour, Equipment, and Facilities.What types of situational restrictions are there?
A few instances from a typical workplace are an unpleasant temperature, a particularly obnoxious or loud coworker in the cubicle next to you, bad lighting, foul aromas, noisy equipment, or a supervisor who is overly demanding.What are some instances of societal restrictions?
To be more precise, we define social constraints as behavioural patterns that present possibilities for and place restrictions on the execution of engineering projects. Social restrictions may take the shape of official procedures like government laws or informal norms like cultural preferences.To know more about constraints, checkout this link:
https://brainly.com/question/26441667
#SPJ1
Which of the activities below does NOT affect the water cycle
Hydroelectricity
Deforestation
Exercising
Greenhouse effect
Answers
Answer:
Exercising would not affect the water cycle.
What is the specific heat capacity of silver metal if 110.00 g of the metal
absorbs 396 J of heat and the temperature rises 15.0°C ?
Answers
Answer:
"0.24 J/g.k" is the appropriate solution.
Explanation:
Mass of substance,
m = 110.00 g
Heat,
q = 396 J
Change in temperature,
ΔT = 15.0°C
Now,
The specific heat will be:
⇒ \(q=ms \Delta T\)
On putting the given values, we get
⇒ \(396=110.00\times s\times 15.0\)
⇒ \(396=s\times 1650\)
⇒ \(s=\frac{396}{1650}\)
⇒ \(=0.24 \ J/g.k\)
density = 11g/cm3 volume = 2cm3 what is the mass
Answers
Density = 11g/cm³, volume = 2cm³. What is the mass ?
Solution:-We know,
\( { \boxed{\bf{\rm \red {Mass \: = \: Density \: × \: Volume}}}} \)
So, Mass = 11g/cm³ × 2cm³
Mass = 22 g
The mass is 22 g. [Answer]the helium is heated from 9.0 °c to 79.0 °c and also expands from a
Answers
When helium is heated from 9.0 °C to 79.0 °C and expands from a volume of 3.50 L to 3.89 L, it is an indication that the process is an isobaric process. The reason for this is that the pressure remains constant throughout the process.
Isobaric processes are also referred to as constant pressure processes. It is a thermodynamic process in which the pressure remains constant while the volume changes. Heat is absorbed by the gas when it is heated, causing its molecules to gain kinetic energy. As the kinetic energy increases, the molecules' movement becomes more erratic, and they begin to collide with each other more frequently. As a result, the distance between them expands, resulting in an expansion in the volume of the gas. The ideal gas law states that PV=nRT where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature in Kelvin (K). In an isobaric process, pressure (P) is constant, and since n, R, and P remain constant, the ideal gas law can be simplified as: V/T = constant. This equation shows that if temperature (T) increases, then volume (V) must also increase in order to keep the constant value intact. In the given problem, the volume increased from 3.50 L to 3.89 L due to the heating of helium from 9.0 °C to 79.0 °C.
For more information on isobaric processvisit:
brainly.com/question/23781022
#SPJ11
PLEASE HURRY !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!! WILL GIVE BRAINLIEST
What type of rock is limestone? Describe how a limestone rock is likely to change over a long period of time.
Answers
Answer:
Over time, the limestone is broken down into its chemical parts and may come back to the surface as volcanic CO2 (in the atmosphere). From the atmosphere, the CO2 might again become part of the biosphere by molluscs or corals absorbing the CO2 to help make their shells
Explanation:
Over a long period of time, we would take a look at the rock "limestone" through the rock cycle. Limestone being a sedimentary rock would be converted to marble, a metamorphic rock if subjected to metamorphic conditions over an extensive period of time.
Compute Reaction Rates for All Seven Trials
Assume that each tablet's mass was 1,000 mg, and remember that you used 0.200 L of water each time.
Compute the reaction rate to the nearest whole number using the formula below.
Reaction Rate
mass of tablet / volume of water
reaction time
3°C
Reaction time = 138.5 sec
Reaction rate =
mg/L/sec
24°C
Reaction time = 34.2 sec
Reaction rate
mg/L/sec
40°C
Reaction time = 26.3 sec
Reaction rate =
mg/L/sec
65°C
Reaction time = 14.2 sec
Reaction rate
mg/L/sec
DONE
Answers
Reaction rate at
3 °C is 36.1 mg/L/sec24 °C is 146.2 mg/L/sec40° C is 190.1 mg/L/sec65 °C is 352.1 mg/L/secWhat is reaction rate?
The rate of a chemical reaction is defined as rate of change in concentration of a reactant or product divided by its coefficient from the balanced equation
Reaction rate = \(\frac{mass of tablet / volume of water }{reaction time}\)
For 3° C:
Reaction rate = \(\frac{1000mg / 0.200L}{138.5 sec}\) = 36.1 mg/L/sec
For 24 °C:
Reaction rate = \(\frac{1000/0.200}{34.2}\) = 146.2 mg/L/sec
For 40°C:
Reaction rate = \(\frac{1000/0.200}{26.3}\) = 190.1 mg/L/sec
For 65 °C:
Reaction rate = \(\frac{1000/0.200}{14.2}\) = 352.1 mg/L/sec
Learn more about reaction rate at https://brainly.com/question/12904152
#SPJ13
describe the molecular stucture of a liquid and add good sciency vocab
Answers
The arrangement and mobility of molecules in a fluid state, controlled by intermolecular interactions, are included in the molecular structure of a liquid.
Molecular structure of liquidsA liquid is made up of a group of particles, usually molecules, that are constantly moving and display intermolecular forces of attraction. These intermolecular forces, including hydrogen bonds, dipole-dipole interactions, and van der Waals forces, are very important in influencing the behavior and characteristics of the liquid.
Although the molecules in a liquid are closely packed, they are not organized in a predictable way like they are in a solid. Instead, they are sufficiently energetic to move past one another, giving rise to a nature that is fluid and shape-adaptive. This property enables liquids to adopt the shape of the container they are contained in.
A liquid's molecular structure is dynamic and always in motion. Although individual molecules are free to move, intermolecular forces they encounter have an impact on how they behave. Depending on the sort of molecules present and their functional groups, these forces' potency and nature can change.
Learn more on liquids here https://brainly.com/question/28554949
#SPJ1
In a living cell which process uses up atp
Answers
Answer:
Metabolism
Explanation:
because energy change takes place
A gas at 65.0°C occupies 4.22 L. If the pressure remains the same, at which
Celsius temperature will the volume be 3.87 L?
Answers
A gas at 65.0°C occupies 4.22 L. If the pressure remains the same, at which Celsius temperature will the volume be 3.87 L out to be 310 degrees
You can leave the pressure out of both sides of the equation since the pressure is constant to make it easy.
so all you have is volume over Temp = new volume over new temp which is we don't know.
4.22 liters over 338 Kelvin = 3.87 liters over T
cross multiply and solve for T which comes out to be 310 degrees K or 37 degrees C
Yes you are right it is 310
Learn more about volume https://brainly.com/question/1578538
#SPJ1
Question 4
Which best describes a chemical reaction that follows the law of conservation of matter?
А
The reactants have the same mass as the products.
B
The reactants have the same density as the products.
С
The products conserve all physical properties of the reactants.
D
The products conserve all chemical properties of the reactants.
Answers
How does the temperature change when a layer of glass is added?
Answers
Answer:
thermal shock
Explanation:
the temperatures inside the glass jar should have continued to increase over time. Internal stresses due to uneven heating. This is also known as “thermal shock”.
In general, the thicker the glass, the more prone it will be to breaking due to the immediate differences in temperature across the thickness of glass.
Borosilicate glass is more tolerant of this, as it has a higher elasticity than standard silicon glass.
You may also note that laboratory test tubes and flasks are made with thinner walls, and of borosilicate glass, when designated for heating.
An eraser is an example of —
Group of answer choices
an insulator
a closed circuit
a conductor
an open circuit
Answers
Answer:
Insulator
Explanation:
An eraser can't conduct electricity, which makes it an insulator.
please answer all parts thank you
Complete the simple analysis of temperature (for which there are always observations of temperature that correspond to the contour values) in Figure 2 for the 75 and 70°F isotherms. The 80°F contour
Answers
Given Figure 2 below shows a set of contour lines for temperature, and the question wants you to complete a simple analysis of temperature. The analysis should be made for the 75 and 70°F isotherms. The 80°F contour is also to be analyzed.
Figure 2 From the image above, we can identify the following contour lines and their values:Contour line C1 is for a temperature of 60°F.Contour line C2 is for a temperature of 65°F.Contour line C3 is for a temperature of 70°F.Contour line C4 is for a temperature of 75°F.Contour line C5 is for a temperature of 80°F.Using the given information, we can then proceed to answer the questions as follows:Analysis for the 75°F isotherm Contour line C4 shows a temperature of 75°F. This means that any point lying on this contour line has a temperature value of 75°F. Therefore, we can conclude that the following regions have a temperature of 75°F:Region A: This region is enclosed by contour lines C3 and C4.
Thus, it has a temperature of 75°F.Region B: This region is enclosed by contour lines C4 and C5. Thus, it has a temperature of 75°F.Analysis for the 70°F isotherm Contour line C3 shows a temperature of 70°F. This means that any point lying on this contour line has a temperature value of 70°F. Therefore, we can conclude that the following regions have a temperature of 70°F:Region C: This region is enclosed by contour lines C2 and C3. Thus, it has a temperature of 70°F.Region D: This region is enclosed by contour lines C3 and C4. Thus, it has a temperature of 70°F.Analysis for the 80°F contourContour line C5 shows a temperature of 80°F. This means that any point lying on this contour line has a temperature value of 80°F. Therefore, we can conclude that the following regions have a temperature of 80°F:Region E: This region is enclosed by contour lines C4 and C5. Thus, it has a temperature of 80°F.
To know more about point lying visit:-
https://brainly.com/question/30174758
#SPJ11
What is the molar mass of an unknown gas with a density of 3.35 g/L at 1.00 atm and 25.0 °C
Answers
Answer:
\(\implies \sf D = \dfrac{PM}{RT}\)
\(\implies \sf 3.35= \dfrac{1 \times M}{ \frac{1}{12} \times 298}\)
\(\implies \sf 3.35= \dfrac{12\times M}{ 298}\)
\(\implies \sf 3.35 \times 298= 12\times M\)
\(\implies \sf 998.3= 12\times M\)
\(\implies \sf M = \dfrac{998.3}{12} \)
\(\implies \bf M = 83.1917 \: \frac{g}{mol} \)
The steady state vital to life is possible because:________
a. the cell cannot convert energy from one form to another.
b. all cells are autotrophic.
c. all reactions are exothermic.
d. the cell continually takes up energy from the environment.
e. all reactions are at equilibrium.
Answers
Answer: The correct answer is e) all reactions are at equilibrium.
Explanation: In order for cellular vitality to develop, it is necessary for it to be in energetic balance with the environment, that is, to give and receive energy with the environment that surrounds it through endothermic or exothermic reactions. That is why the development of life is considered a system that constantly exchanges with the environment. In turn, that the cell unit maintains a balance with the environment causes homeostasis to occur among the whole organism.
Determine the mass in grams of 3.60 mol of H2 SO4
Answers
Answer:
Explanation:
There are 353 grams of H2SO4 in 3.60 moles of H2SO4.
design a synthesis of 2-hexanone from compounds containing four carbons or fewer.
Answers
One possible synthesis of 2-hexanone from compounds containing four carbons or fewer could start with the compound ethylacetoacetate (EAA), which has three carbons.
EAA can be reacted with ethyl iodide in the presence of a strong base, such as sodium hydride, to yield the compound ethyl 3-oxobutanoate. This compound has four carbons and can be further reacted with a Grignard reagent, such as methylmagnesium bromide, to form the intermediate compound 3-hexanol. This compound can then be oxidized with a strong oxidizing agent, such as potassium permanganate, to form 2-hexanone, which has six carbons. The final product can be purified using distillation or other separation techniques. This synthesis involves multiple steps and requires careful handling of reactive chemicals, but can yield high purity 2-hexanone from simple starting materials.
To synthesize 2-hexanone from compounds containing four carbons or fewer, we can use a three-step process. First, perform a Grignard reaction between ethyl magnesium bromide (CH3CH2MgBr) and butanone (CH3CH2COCH3) to form a tertiary alcohol. Next, conduct an oxidation of the tertiary alcohol using a strong oxidizing agent such as chromium trioxide (CrO3) to create a ketone, 2-hexanone (CH3CH2CH2COCH2CH3). This method efficiently combines smaller carbon compounds to produce the desired target molecule, 2-hexanone, with the given restrictions on starting materials.
To know about hexanone :
https://brainly.com/question/28836525
#SPJ11
Why does ninhydrin stain the skin blue? a. Skin contains amino acids. b. Ninhydrin is blue-colored c. Ninhydrin turns blue when warmed
Answers
Option A, Ninhydrin is a chemical that is used to detect the presence of amino acids in a sample. It reacts with the amino acids in a sample, such as skin, to form a complex that is blue in color.
Ninhydrin is a chemical that is used to detect the presence of amino acids in a sample. It reacts with the amino acids in a sample, such as skin, to form a complex that is blue in color. This is because when Ninhydrin reacts with an amino acid it forms a complex with the nitrogen in the amino group, and this complex is blue in color. It is also commonly used in forensic science to detect fingerprints, as fingerprints contain amino acids from the oils and sweat on the skin. The blue coloration of the skin is an indication of the presence of amino acids, which are found in many biological molecules such as proteins and enzymes.
Learn more about amino acid here:
https://brainly.com/question/24106148
#SPJ4
can someone help me calculate the average speed??

Answers
Answer:
3 meters per second
Explanation:
Average speed = total distance / total time
We know that total distance = 45 meters and total time = 15 seconds, hence, average speed = 45 meters / 15 seconds = 3 meters per second.
Answer:
V=S/t
Explanation:
V=?
S= 45meters
t=15sec
V=S/t
V=45/15
V=3m/sec
List at least 3 physical properties if water.
Answers
Answer:
Appearance: Water is a colourless, odourless and tasteless liquid in its natural state. The crystal structure of water is hexagonal.
Density: The density of water is about 1 gm/cc and it varies with temperature in an undefined pattern. In solid state, the density remains close to 0.9gm/cc.
Viscosity: Viscosity is explained by the resistance to deformation at a given rate. In other words, the thickness of the liquid - eg, syrup or water. Viscosity of water is 0.89 cP.
Explanation:
_____ are reaction where molecules are broken apart to make new substances
Answers
Decomposition are reaction where molecules are broken apart to make new substances
A chemical reaction known as a decomposition reaction involves the breakdown of a compound into its simpler components, compounds, or molecules. The opposite of a synthesis reaction, which combines two or more compounds, is this kind of reaction.
Decomposition reactions include the breakdown of hydrogen peroxide into water and oxygen gas, the breakdown of baking soda into sodium carbonate, water, and carbon dioxide, and the breakdown of glucose into carbon dioxide and water during cellular respiration. The general formula for a decomposition reaction is AB A + B, where AB is the compound that is decomposed into A and B.
Know more about decomposition reactions:
https://brainly.com/question/14024847
#SPJ11
if you were given an unknown sample, how would you determine if it was a suspension, colloid or homogeneous solution?
Answers
The Tyndall effect can be used to detect whether an unknown sample is a suspension, colloid, or homogeneous solution.
Light can always travel through a solution, and there is never any general light scattering. Between solutions and suspensions, a colloid exists. By diffusing the incoming light, it produces the Tyndall effect. A suspension is hazy and contains visible suspended particles. The suspensions obstruct the passage of light.
A combination that is heterogeneous and has some of its particles settle out as it stands is called a suspension. Therefore, if a beam of light is passed through your sample and it scatters, it is either a suspension or a colloid. It is a solution if it doesn't scatter.
To know more about tyndall effect, refer:
https://brainly.com/question/3284136
#SPJ4
A combustion reaction a. normally requires oxygen b. requires a fuel c. gives off energy d. all of the above
Answers
Combustion reactions require oxygen, a fuel, and give off energy.
Combustion reactions, also known as burning, are chemical reactions that occur when a fuel combines with oxygen in the presence of an ignition source. The main answer to the question is that combustion reactions require all of the mentioned components: oxygen, a fuel, and they give off energy.
Oxygen is a crucial component in combustion reactions because it acts as an oxidizer. It combines with the fuel, which can be any combustible material such as wood, gasoline, or natural gas. The presence of oxygen enables the fuel to undergo oxidation, releasing energy in the form of heat and light.
Without a fuel source, combustion cannot occur. The fuel provides the carbon and hydrogen atoms necessary for the reaction. Different fuels have varying energy content and combustion characteristics, which can affect the intensity and efficiency of the reaction.
When a fuel and oxygen react, a chemical reaction takes place, resulting in the release of energy. This energy is in the form of heat and light, which is why combustion reactions often produce flames. The energy released during combustion can be harnessed for various purposes, such as heating, cooking, or powering engines.
In summary, combustion reactions normally require oxygen as an oxidizer, a fuel source to provide the necessary atoms, and they give off energy in the form of heat and light. These reactions are essential for various practical applications and play a vital role in our daily lives.
Learn more about combustion reactions
brainly.com/question/14335621
#SPJ11
What are the current and or future uses of genetically modified strawberries
Answers
In the future, genetically modified strawberries may become more widely available if they pass regulatory approvals and are deemed safe for consumption. They could potentially provide benefits such as reduced pesticide use, longer shelf life, and improved nutrition. However, there are also concerns about the environmental impact and potential health risks associated with genetically modified crops, which will need to be addressed before they can be widely adopted.
Describe the relationship between thermal energy and temperature of a substance.
help me pls :D
Answers
Overall, when temp goes up, thermal energy goes up, which leads to an increase in kinetic energy.
Find the period 2 elements (atomic numbers #3-10) and the period 3 elements (#11 - 18). Do period 2 and period 3 have the same trend?
Period 2 elements are Lithium (Li), Berillium (Be), Boron (B), Carbon (C), Nitrogen ( N), Oxygen (O), Fluorine (Fe), Neon (Ne). Period 3 elements are Sodium (Na), Magnesium (Mg), Aluminum (AI), Silicon (Si), Phosphorus (P), Sulfur (S), Chlorine (CI, Argon, (Ar)
do period 2 and period 3 have the same trend ? (if they do can you explain a bit ?)
(brainly !!)
Answers
Period 2 and period 3 elements do not have the same trend in terms of their electronic configurations. Period 2 elements have 2 electron shells and period 3 elements have 3 electron shells.
How does period 3 and period 2 elements differ?The elements in each period have one more electron shell than the elements in the previous period. As a result, the elements in each period have a different number of valence electrons, which affects their chemical properties.
As the period increases, the elements in the period become more metallic. The elements of Period 2 are generally non-metals, and the elements of Period 3 are generally metals.
Find out more on period 2 and 3 elements here: https://brainly.com/question/18878801
#SPJ1