Answers
Answer:
0.32 grams
Explanation:
To go from moles to grams (I assume you meant mass instead of weight) you start by finding the molar mass. For O2, the molar mass is 32, because there are two oxygen atoms so 16 grams multiplied by 2.
After that, you take your given moles and multiply it by the grams per mole. The moles cancel each other out and you get your value in grams. I have attached my work below.
Hope this helps! :^)

Related Questions
Please help, its due today! I'll also make you brainiest (put them in an order that's simple, look at the picture and you'll see what I mean) Thank you and God bless! <33
On beaches there are often areas of grassy dunes where people are prohibited from walking. How do these protected areas preserve ecosystem services? Use the graphic organizer to categorize the following as either examples of land reclamation of protecting biodiversity.

Answers
Answer:
Preventing erosion – Land Reclamation
Protecting nesting areas – Protecting Biodiversity
Preventing littering – Land Reclamation
Preventing habitat disruption – Protecting Biodiversity
Protecting native species – Protecting Biodiversity
Preventing contamination of soil – Land Reclamation
Explanation:
I really hope I'm right! I tried my hardest, please give me brainliest :)
have a good day!
a central atom with 3 bonding domains and 2 nonbonding domains has geometry. both nonbonding domains are in the plane since this allows the maximum average distance between nonbonding domains.
Answers
Tetrahedral is the shape of the molecule. Three bonding domains and two nonbonding domains exist for a centre atom that possesses geometry. Two nonbonding domains are present in the plane.
A number is said to be tetrahedral if it can be represented by the pyramidal shape known as the tetrahedron, which has three sides and a triangle base. The nth tetrahedral number is equivalent to the first n triangular numbers added together. A molecule is defined as a collection of two or more atoms held together by the attractive forces known as chemical bonds; depending on the context, the term may or may not include ions that fit this criteria. Atoms, the smallest unit of matter, exhibit the properties of a chemical element, such as hydrogen.
Learn more about molecules here
https://brainly.com/question/19922822
#SPJ4
The complete question is-
A central atom with 3 bonding domains and 2 nonbonding domains has geometry. both nonbonding domains are in the plane since this allows the maximum average distance between nonbonding domains. The shape of the molecule is ?
Which of the statements listed below is negative aspect of a volcanic eruption?
A. generating geothermal energy.
B. lava flows and lahars clear areas of woodland or agriculture,
C. the dramatic scenery created by volcanic eruptions attracts tourist.
D. lava and ash deposited during a volcanic eruption breaks down to provide valuable
nutrients for the soil.
CANO
Answers
Answer:
C . the dramatic scenery created by volcanic eruptions attracts tourists.
The statement 'lava flows and lahars clear areas of woodland or agriculture' is a negative aspect of a volcanic eruption (Option B).
A volcanic eruption refers to the expulsion of lava and dangerous gases from a volcano.These volcanic eruptions can destroy large areas including lands for agriculture or woodland.A lahar can be defined as a mix of cold/warm water and fragments of sediments (rocks) that flow fast down the slope of the volcano.In conclusion, the statement 'lava flows and lahars clear areas of woodland or agriculture' is a negative aspect of a volcanic eruption (Option B).
Learn more in:
https://brainly.com/question/1622004
The number of transition elements in the 1, 2" and 3rd transition series... a) 9 b) 30 c) 10 d) 27
Answers
Answer: There are
30 elements for the 1st transition series, 10 elements for the 2nd transition series, 3rd transition series is not listed as one of the given options, but it consists of 14 elements.Explanation:
1st transition series: The 1st transition series spans the elements from Scandium (Sc) to Zinc (Zn) in the periodic table.These elements fill the 3d orbitals. Since there are 10 elements in each period of the d-block, the 1st transition series consists of 10 elements. 2nd transition series: The 2nd transition series includes the elements from Yttrium (Y) to Cadmium (Cd) in the periodic table. These elements fill the 4d orbitals. Similar to the 1st transition series, there are 10 elements in each period of the d-block, so the 2nd transition series also consists of 10 elements.3rd transition series: The 3rd transition series includes the elements from Lanthanum (La) to Mercury (Hg) in the periodic table. These elements fill the 5d orbitals. In the 5d orbital, there are a total of 10 elements in each period of the d-block. However, the 3rd transition series does not include all 10 elements of the 5d block. It includes 14 elements from Lanthanum (La) to Lutetium (Lu). Therefore, the 3rd transition series consists of 14 elements.What is the standard enthalpy of formation of liquid methylamine (CH3NH2) ?C(s)+O2(g) -> CO2(g); ?H=-393.5 kJ 2H2O(l) -> 2H2(g)+O2(g); ?H=571.6 kJ N2(g)+O2(g) -> NO2(g); ?H=33.10 kJ 4CH3NH2(l)+13O2(g) -> 4CO2(g)+4NO2(g)+10H2O(l); ?H=-4110.4 kJ The calculated answer is -47.3 kJ/mol. Show the work to confirm or deny the answer
Answers
Answer:
ΔH =-47.3kJ
Explanation:
You must know standard enthalpy is defined as change of enthalpy during the formation of 1 mole of the substance from its constituent elements
You can find the standard enthalpy of any reaction from the sum of another similar reactions (Hess's law) as follows:
For methylamine, CH₃NH₂ is:
C(s) + 5/2 H₂(g) + 1/2 N₂(g) → CH₃NH₂ (l)
1. C(s) + O₂(g) → CO₂(g); ΔH=-393.5 kJ
2. 2H₂O(l) → 2H₂(g) + O₂(g); ΔH=571.6 kJ
3. 1/2N₂(g) + O₂(g) → NO₂(g); ΔH=33.10kJ
4. 4CH₃NH₂(l) + 13O₂(g) → 4CO₂(g) + 4NO₂(g) + 10H₂O(l); ΔH=-4110.4 kJ
The sum of (1)+(3) produce:
C(s) + 2O₂(g) + 1/2N₂(g) → CO₂(g) + NO₂(g) ΔH=-393.5kJ + 33.10kJ = -360.4kJ
-5/4 (2):
C(s) + 13/4O₂(g) + 1/2N₂(g) + 5/2H₂(g) → CO₂(g) + NO₂(g) + 5/2 H₂O(l)
ΔH= -360.4kJ -5/4 (571.6kJ) = -1074.9kJ
And this reaction -1/4 (4):
C(s) + 5/2 H₂(g) + 1/2 N₂(g) → CH₃NH₂(l)
ΔH= -1074.9kJ -1/4(-4110.4kJ)
ΔH =-47.3kJNow, you can confirm the calculated answer!
Cómo se forma un enlace polipeptido?
Answers
Water has a density of 2g/ml. What is the mass of the water if it fills a 10ml container
Answers
20 Grams of Water.
Grams : mL
2 : 1
4 : 2
6 : 3
8 : 4
10 : 5
12 : 6
14 : 7
16 : 8
18 : 9
20 : 10
Answer:
20 grams
Explanation:
mass = Density × Volume
= M = 2g/ml × 10ml
= 20g
Help!!!
Question: What is the correct order of the particles that give texture to soil from smallest to largest?
Options:
A: Clay, sand, silt
B: silt, sand, clay
C: clay, silt, sand
D: sand, silt, clay
Answers
The correct order of the particles that give texture to the soil from smallest to largest is clay, silt, and sand; option C.
What is soil texture?Soil texture refers to a physical classification of the component and types of soils based on their physical texture either as coarse or fine particles.
There are several types of soils and these various types of soils have different textures.
The types of soils and their arrangement based on increasing particle size are as follows:
clay soil - this is the finest particle soil typesilt - this is the next soil type in terms of texturesandy soil - this is the largest of the three soil types in terms of size and texture.Learn more about soil texture at: https://brainly.com/question/8513717
#SPJ1
4. One molecule of propanol contains a total of
flonsoona
(1) one -OH group
(2) two -CH3 groups
(3) three -OH groups
(4) three -CH3 groups
Answers
One molecule of propanol contains only one -OH group and not three -OH groups or three -CH3 groups.
The -OH group is attached to the central carbon atom and makes propanol a useful solvent and intermediate in organic chemistry.Propanol is a colorless liquid that belongs to the family of alcohols. It has the formula C3H8O, and it contains three carbon atoms, eight hydrogen atoms, and one hydroxyl group (-OH) attached to one of the carbons. One molecule of propanol contains only one -OH group, which is attached to the central carbon atom.
Thus, option (2) is the correct answer, and the other options are incorrect.The -OH group in propanol is responsible for its unique chemical and physical properties. It makes propanol soluble in water and other polar solvents and gives it a high boiling point of around 97°C. The hydroxyl group can also participate in chemical reactions, such as esterification, dehydration, oxidation, and reduction. For example, propanol can be oxidized to form propanal and then propanoic acid, which is a useful synthetic intermediate for many organic compounds.Apart from the -OH group, propanol also contains two other functional groups called methyl groups (-CH3). These are attached to the two carbon atoms adjacent to the central carbon. However, the question only asks about the number of -OH groups in propanol, so the methyl groups are irrelevant.
for such more questions on propanol
https://brainly.com/question/30086385
#SPJ8
Which impact is associated with sustainable agriculture?
O less cropland is lost
O aquifers are depleted more quickly
O soil erodes more quickly
O farmland becomes overgrazed
Answers
Less cropland is lost impact is associated with sustainable agriculture
Agriculture is the arena's biggest industry. It employs multiple billion humans and generates over $1.three trillion bucks really well worth of meals annually. Pasture and cropland occupy round 50 percentage of the Earth’s liveable land and offer habitat and meals for a large number of species..
When agricultural operations are sustainably managed, they could keep and repair crucial habitats, assist defend watersheds, and enhance soil fitness and water quality. But unsustainable practices have severe influences on humans and the environment.
The want for sustainable useful resource control is more and more more urgent. Demand for agricultural commodities is growing hastily as the arena's populace grows. Agriculture’s deep connections to the arena economy, human societies and biodiversity make it one of the maximum essential frontiers for conservation across the globe.
learn more sustainable agriculture here https://brainly.com/question/26177941
#SPJ1
3. Beaker A (Heat Meter) tells us how much heat energy left the hot
plate and entered into Experiment Beaker C. One calorie of energy
is the amount of heat required to increase the temperature of 1 ml
of water 1°C. How much did the temperature change in Beaker A?
°C. (Subtract the starting temperature if it was above 0°C.)
4. Now, calculate the total calories of heat recorded by the Heat Meter
(Beaker A) considering both the amount of water in it and the
temperature change of the water.
calories
5. The answer to question #4 is the amount of energy to vaporize 10 ml
of water in Beaker C. How many calories would be required to
evaporate only 1 ml of water?
calories
6. Based on your results, discuss how effective is the evaporation of
water at removing excess heat from your body (sweating).
L
Answers
540 calories per ml or g of water required to evaporate only 1 ml of water.
About 540 calories per ml or g of water are needed to convert one gram of water that is present in liquid state into vapor form under normal pressure. The evaporation of water at removing excess heat from our body is very effective in lowering the temperature of our body and in maintaining homeostasis.
Our body removes heat with the help of water that is move from the small openings present on our skin in the form of sweats so we can say that evaporation of water at removing excess heat from our body is very effective.
Learn more: https://brainly.com/question/24725199
the questions are in the picture.

Answers
a) The wavelength of the calcium is 620 nm
b) The energy of the calcium is 3.2 * 10^-19 J
c) Electronic configuration of calcium is \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^2\)
d) The shorthand configuration is [Ar] 4s².
What is the light?We know that the flame test is the kind of test that we can be able to use to know the ion that has been present in the sample that we are trying to analyze. When the ample is moistened and exposed to light, we would see a color that corresponds to a given wavelength.
We know that the wavelength of the calcium light can be obtained as 620 nm and that we can see this to be the orange - red light of the calcium shown.
The energy of the light is given from;
E = hc/λ
E = energy
h = Plank's constant
c = speed of light
λ = wavelength
Then we have;
E = 6.6 * 10^-34 * 3 * 10^8/ 620 * 10^-9
E = 3.2 * 10^-19 J
Learn more about calcium:https://brainly.com/question/29597119
#SPJ1
The term "cycle" is used in Water Cycle because ?
Answers
Answer:
because cycle is a prosess that goes through multiple stages
Explanation:
.
The fossilized remains of a plant were found at a construction site. The fossilized remains contain 1/32 the amount of carbon-14 that is present in a living plant.
Determine the approximate age of these fossilized remains.
Answers
Answer:
28645 years
Explanation:
Given the formula;
0.693/t1/2 = 2.303/t log (No/N)
Given that N = 1/32 No
Note;
t1/2 = half life of Carbon-14
t = time required for N amount of carbon -14 to remain= 5,730 years
No= amount of carbon 14 initially present
N = amount of carbon-14 after time t
Substituting values;
0.693/5,730 = 2.303/t log (No/1/32No)
0.693/5,730 = 2.303/t log 32
1.21 * 10^-4 = 3.466/t
t = 3.466/1.21 * 10^-4
t = 28645 years
Any preserved imprints, remains and traces of once a living organism from history are called fossils. By utilizing the radioactive property of organic compounds one can determine the age of an object.
The approximate age of the fossilizes remain is 28645 years.
This can be estimated as:
The formula used will be:
\(\dfrac{0.693}{\dfrac{t1}{2}}= \dfrac{2.303}{t} log (\dfrac{No}{N})\)
Where,
Half-life of Carbon-14 =\(\dfrac{t1}{2}\) Time required (t) for N amount of carbon -14 to remain= 5,730 yearsAmount of carbon 14 initially present = \(N_{o}\)Amount of carbon-14 after time t = NGiven,
N = \({\dfrac{1}{32} No\)
Replacing values in formula:
\(\dfrac{0.693}{5,730} & = \dfrac{2.30}{t} log \:({{\dfrac{No}{\dfrac{1}{32} No}})\)
\(\dfrac{0.693}{5,730} & = \dfrac{2.30}{t} \;log 32\)
\(1.21 \times 10^{-4} = \dfrac{3.466}{t}\)
\(t = \dfrac{3.466}{1.21} \times 10^{-4}\)
\(t = 28645 \:\text{years}\)
Therefore, the approximate age of the fossil is 28645 years.
To learn more about fossils and their age follow the link:
https://brainly.com/question/8917701
what is the mass of insoluble calcium phosphate produced from .555 grams of calcium chloride
Answers
Answer:
0.518 g
Explanation:
Step 1: Write the balanced equation
3 CaCl₂ + 2 H₃PO₄ ⇒ Ca₃(PO₄)₂ + 6 HCl
Step 2: Calculate the moles corresponding to 0.555 g of CaCl₂
The molar mass of CaCl₂ is 110.98 g/mol.
0.555 g × 1 mol/110.98 g = 5.00 × 10⁻³ mol
Step 3: Calculate the moles of Ca₃(PO₄)₂ produced
5.00 × 10⁻³ mol CaCl₂ × 1 mol Ca₃(PO₄)₂/3 mol CaCl₂ = 1.67 × 10⁻³ mol Ca₃(PO₄)₂
Step 4: Calculate the mass corresponding to 1.67 × 10⁻³ moles of Ca₃(PO₄)₂
The molar mass of Ca₃(PO₄)₂ is 310.18 g/mol.
1.67 × 10⁻³ mol × 310.18 g/mol = 0.518 g
4. How many grams is 3 moles of H₂O?
Answers
Answer:
1.67
Explanation:
Mass÷mr=moles
3÷18=1.67
How many significant figures
are in this number?
5.67 x 10^2
Answers
Answer:
3
Step-by-step explanation:
Hence, the given number has 3 significant figures.
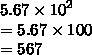
Coffee shops sell paper coffee
cups with cardboard sleeves
for customer safety. Why?

Answers
The cardboard sleeves reduce the heatness from the coffee cup. So then the cosumer does not get a burn.
QUESTION 3
List the two ways of determining the chloride content of water
Answers
After removing the particles, the solution's chloride content can be assessed using various techniques, such as titration and photometry.
What is the importance of chloride determination?One of the most prevalent inorganic anions in water and wastewater is chloride, which forms the chloride (Cl-) ion. Sodium chloride, a specific food item that passes through the digestive tract undigested, has a higher chloride concentration in wastewater than in raw water. Due to salt water leaking into the sewage system, coast chloride may be present in high concentrations along the sea. Additionally, industrial processes may cause it to rise.The salty taste caused by chloride concentration in drinkable water varies and depends on the water's chemical makeup. If sodium cation is present, some waters with 250 mg/L Cl- may have a flavor that can be identified as salty. On the other hand, when calcium and magnesium cations predominate, the typical salty taste may be missing in fluids containing as much as 1000 mg/L. A high chloride level may injure developing plants, steel pipelines, and structures.The salinity of various water sources can be determined using the detected chloride ions. It is a crucial parameter for brackish water (or seawater or industrial brine solution) because it indicates how much desalting of the apparatus is needed. Additionally, it affects the COD calculation, necessitating a modification based on the amount present or adding a complexing agent like HgSO4. Additionally, column studies that predict the fate of various pollutants in soil and liquid media use chloride ions as tracer ions.After removing the particles, the solution's chloride content can be assessed using various techniques, such as titration and photometry.
To learn more about chloride, refer to:
https://brainly.com/question/24490939
#SPJ9
During the chlorination of silicon, chlorine gas is reacted with solid silicon to form silicon tetrachloride.
Si(s) + 2Cl2(g) + heat ↔ SiCl4(g)
Which WILL NOT cause the equilibrium of the reaction to shift towards the products?
Question 18 options:
adding a catalyst
increasing the pressure
increasing the temperature
Increasing the mass of both reactants and products by 20%
Answers
Adding a catalyst will not cause the equilibrium of the reaction to shift towards the products.
A catalyst is a substance that speeds up the rate of a chemical reaction without being consumed in the reaction. It does not affect the position of the equilibrium or the relative amounts of reactants and products at equilibrium.
In the given reaction, Si(s) + 2Cl₂(g) + heat ↔ SiCl₄(g), the forward reaction is exothermic, which means it releases heat. According to Le Chatelier's principle, an increase in temperature will shift the equilibrium towards the reactants side in order to counteract the increase in heat. Therefore, increasing the temperature will shift the equilibrium towards the left, reducing the yield of silicon tetrachloride.
Increasing the pressure of the system will also shift the equilibrium towards the side with fewer gas molecules, which is the products side. In this case, increasing the pressure will shift the equilibrium towards the products, increasing the yield of silicon tetrachloride.
Increasing the mass of both reactants and products by 20% will not affect the position of the equilibrium either, as it does not change the ratio of the concentrations of the reactants and products.
To learn more about equilibrium of the reaction, here
https://brainly.com/question/15118952
#SPJ1
If the initial temperature of an ideal gas at 2.250 atm
is 62.00 ∘C,
what final temperature would cause the pressure to be reduced to 1.700 atm?
Answers
P1V1/T1 = P2V2/T2
where P1, V1, and T1 are the initial pressure, volume, and temperature, and P2, V2, and T2 are the final pressure, volume, and temperature.
We can assume that the volume of the gas is constant, so V1 = V2.
Converting the initial conditions to SI units:
P1 = 2.250 atm * 101.325 kPa/atm = 228.04 kPa
T1 = 62.00 + 273.15 = 335.15 K
Converting the final conditions to SI units:
P2 = 1.700 atm * 101.325 kPa/atm = 172.24 kPa
Solving for T2:
P1/T1 = P2/T2
T2 = P2 * T1 / P1
T2 = 172.24 * 335.15 / 228.04
T2 = 252.4 K
Converting the final temperature to Celsius:
T2 = 252.4 - 273.15 = -20.8 °C
Therefore, the final temperature that would cause the pressure of the ideal gas to be reduced to 1.700 atm is -20.8 °C
How do I go about solving a Nuclear Equation?

Answers
Answer:
How do amoeba respire.
How do plants respire.
(1) Solution A and B are separated by a membrane that is permeable to Mg2 and impermeable to Cl-. Solution A contains 0.1 mM MgCl2, solution B contains 100 mM MgCl2. Mg2 will be at electrochemical equilibrium when solution A is around ____________ mV. 180 mV - 180 mV 90 mV - 90 mV 60 mV - 60 mV
Answers
Answer:
90 mV
Explanation:
Faraday constant, F = 96487
Gas constant, R = 8.314
Number of mole of electron transferred, Z = 2
MgCl in solution A, = 0.1 mm
MgCl in solution A, = 100 mm
Using the relation :
E=(RT/ZF) In(Mg out/Mgin)
E = (8.314*298) / (2*96485) * In(100/0.1)
E = (2477.572 / 192970) * 6.9077552
E = 0.0128391 * 6.9077552
E = 0.0886897 V
E = 88.68 mV (approximately 90 mV)
Which of the following is NOT true about one mole?
the number of atoms in 1 mole of carbon equals the number of atoms in 1 mole of boron
O 12 g of carbon equals one mole of carbon atoms
the mass of 1 mole of carbon atoms equals the mass of 1 mole of boron atoms
one mole contains 6.02 x 10
23
particles
Answers
Mass=molesx molar mass
And the molar masses of these two elements are different so their masses aren’t equal
The number of atoms in 1 mole of carbon equals the number of atoms in 1 mole of boron is false because the molar mass of carbon is different from that of boron. Option A is correct.
The number of moles of an element is expressed as the ratio of the mass of the substance to its molar mass.Moles = Mass/Molar massThis shows that the number of moles of the substance is dependent on the molar mass of the substance.
From the listed option the number of atoms in 1 mole of carbon equals the number of atoms in 1 mole of boron is false because the molar mass of carbon is different from that of boron
Learn more on moles here: https://brainly.com/question/24256751
Consider the reaction described by the following chemical equation.
2 HN3(1) + 2 NO(g) — H2O2(1) + 4 N, (g)
What is the enthalpy change associated with the production of 1 mol of H, O, if a reaction that produces
2.50 g of H, O, releases 65.9 kJ of heat?
Answers
Answer:
Q = -897 kJ/mol
Explanation:
From the given information:
The heat released Q = -65.9 kJ
To start with the molar mass of \(H_2O_2\) = 2 × (molar mass of H) + 2 × (molar mass of O)
= (2 × 1.008) + (2 × 16.0 )
= 34.016 g/mol
However, given that:
mass of \(H_2O_2\) 2.50 g
The number of moles of \(H_2O_2\) = \(\dfrac{mass}{molar \ mass}\)
\(= \dfrac{2.5}{34.016}\)
\(= 7.349 \times 10^{-2} \ mol\)
Finally; Using the formula:
\(\Delta H = \dfrac{Q}{number \ of \ moles}\\ \\ Q = \dfrac{-65.9 \ kJ}{7.349 \times 10^{-2} \ mol}\)
Q = -897 kJ/mol
In the laboratory, a student is directed to prepare a 0.359 M solution of red #40 dye in a 250.0mL volumetric flask. The molecular mass of red #40 is 496.42 g/mol. How many grams of solid dye should the student weigh out to prepare the solution?
Answers
ANSWER
The mass of solid dye the student will use to prepare the solution is 178.15 grams
STEP-BY-STEP EXPLANATION:
What to find? The grams of the solid dye
Giving parameters
The mole of red #40 dye = 0.359 M
The molecular mass of red#40 = 496.42g/mol
The grams of the red#40 dye can be calculated by using the below formula
\(\begin{gathered} \text{Mole = }\frac{\text{ reacting mass}}{\text{molar mass}} \\ \text{Introducing cross multiply} \\ \text{ reacting mass = mole x molar mass} \\ \text{ Reacting mass = 0.359 x 496.24} \\ \text{ reacting mass = 178.15 grams} \\ \end{gathered}\)Hence, the mass of solid dye the student will use to prepare the solution is 178.15 grams
What Is a Homogeneous Mixture? Definition and Examples
Answers
A homogeneous mixture is one that has a consistent chemical make-up. Homogeneous mixtures have only one phase, according to their definition, composition, and characteristics.
Examples. A combination with a uniform composition is said to be homogenous. Because the particles are distributed evenly, these mixtures have a consistent composition. There is only one phase to them. They are made up of molecular or atomic level ingredients and do not divide into layers. In colloquial words, homogeneous mixes are frequently referred to as solutions. The example that follows is one of the simplest. dissolve sugar in water. A homogenous mixture is a combination of ingredients combined so thoroughly that the separate ingredients are invisible. There will be the identical amounts of each ingredient in every sample of the mixture. Mixtures that are homogeneous can be solid, liquid, or gaseous.
Learn more about homogeneous mixture here
https://brainly.com/question/24898889
#SPJ4
I need help figuring it out the answers were wrong I put in

Answers
PLEASE HELP QUICKLY!!!
HI gas is removed from the system
at equilibrium below. How does the
system adjust to reestablish
equilibrium?
51.8 kJ + H₂(g) + 1₂(g) = 2HI(g)
A. The reaction shifts to the right (products) and the concentrations
of I, and H₂ decrease.
B. The reaction shifts to the left (reactants) and the concentrations
of H₂ and I increase.
C. The reaction shifts to the right (products) and the concentrations
of I, and H₂ increase.
D. The reaction shifts to the left (reactants) and the concentration of
HI increases.

Answers
Answer:
A. The reaction shifts to the right (products) and the concentrations of I and H₂ decrease.
Explanation:
If gas is removed from the system at equilibrium, the system will try to compensate for the loss by shifting the reaction in a direction that produces more gas molecules. This is known as Le Chatelier's principle, which states that a system at equilibrium will respond to a disturbance by shifting in a way that minimizes the effect of the disturbance.
In this case, since gas is being removed from the system, the reaction will shift to the side that produces more gas molecules. Looking at the balanced equation, we can see that 2HI(g) has a greater number of gas molecules compared to H₂(g) and I₂(g). Therefore, the system will shift to the right (products) to produce more HI(g) and reestablish equilibrium.
A solution containing 0.026 moles of H2O2 at 25.0 °C is placed in a coffee cup calorimeter and
allowed to decompose completely according to the thermochemical equation shown below. The final temperature of the solution is 44.9 °C. Calculate the enthalpy of the reaction shown, in kJ/mol. The mass of the solution is 30.0 g and the specific heat capacity of the solution
is 4.18 J/g°C.
CHEMICAL Formula is in the photo

Answers
The enthalpy (in kJ/mol) of the reaction, given that 0.026 moles of H₂O₂ at 25.0 °C is placed in a coffee cup calorimeter is 95.98 KJ/mol
How do i determine the enthalpy of the reaction?First, we shall determine the heat energy of the reaction. Details below:
Mass of solution = 30 gInitial temperature of statue (T₁) = 25 °CFinal temperature of statue(T₂) = 44.9 °CChange in temperature (ΔT) = 44.9 - 25 = 19.9 °C Specific heat capacity of solution (C) = 4.18 J/gºC Heat energy (Q) =?Q = MCΔT
Q = 30 × 4.18 × 19.9
Q = 2495.46 J
Finally, we shall determine the enthalpy of the reaction. Details below:
Heat absorbed (Q) = 2495.46 J = 2495.46 / 1000 = 2.49546 KJMole of H₂O₂ (n) = 0.026 moleEnthalpy of reaction (ΔH) =?Q = n × ΔH
2.49546 = 0.026 × ΔH
Divide both sides by 0.026
ΔH = 2.49546 / 0.026
ΔH = 95.98 KJ/mol
Thus, the enthalpy of reaction is 95.98 KJ/mol
Learn more about enthalpy change:
https://brainly.com/question/24170335
#SPJ1