Answers
Answer:
1.0 M
Explanation:
Reaction equation;
KOH(aq) + HCl(aq) -----> KCl(aq) + H2O(l)
Concentration of acid CA = ?
Concentration of base CB = 1.0 M
Volume of base VB = 25.60 - 0.50 = 25.1 ml
Volume of acid VB = 25.0 ml
Number of moles of acid NA = 1
Number of moles of base NB =2
CAVA/CBVB =NA/NB
CAVANB = CBVBNA
CA = CBVBNA/VANB
CA = 1 * 25.1 * 1/25.0 *1
CA = 1.0 M
Related Questions
what can a wave be described as
Answers
Answer:
wavy, a feel of water, smooth and costy
Explanation:
The energy released per gram of material is __________.
A much larger in nuclear fusion reactions than in chemical reactions
B much smaller in nuclear fusion reactions than in chemical reactions
C the same amount in nuclear fusion reactions as it is in chemical reactions
D insignificantly larger in nuclear fusion reactions than in chemical reactions
Answers
Answer:
I'm pretty sure the answer is C.
Which of the following statements describes the cause of ice ages
Answers
What causes the changes in the ice ages is when the earth's orbits tilts.
What is the cause of the ice ages?
The ice ages are caused by changes in Earth's orbit and tilt, which affect the amount of solar radiation received by the planet. These changes alter the climate and trigger the growth and retreat of ice sheets.
We know that the changes in the climates is responsible for most of the changes that we can see in the universe as we have come to experience it today.
Thus the earth's orbits is what does control the changes in the ice ages.
Learn more about the ice ages:https://brainly.com/question/29417575
#SPJ1
Describe how temperature changes as you travel up Through earths atmosphere
Answers
Answer:The troposphere is heated from the ground, so temperature decreases with altitude. Because warm air rises and cool air sinks, the troposphere is unstable. In the stratosphere, temperature increases with altitude. The stratosphere contains the ozone layer, which protects the planet from the Sun's harmful UV radiation.
Explanation:
Make an argument about the following claim: Exothermic reactions only release thermal energy
Answers
Exothermic reactions only release thermal energy, raising the temperature of the immediate environment. The environment is cooled through an endothermic process that absorbs heat.
What is an exothermic reaction ?An exothermic process is one in which energy is given off as heat or light. In contrast to an endothermic process, which draws energy from its surroundings, an exothermic reaction transfers energy into the environment.
The most exothermic reaction is the burning of methane because it generates a significant quantity of heat.
Thus, Exothermic reactions only release thermal energy.
To learn more about an exothermic reaction, follow the link;
https://brainly.com/question/10373907
#SPJ9
The reaction X - Y has a reaction enthalpy of - 64 kJ moJ-1 and an activation energy of 22 kJ moJ^-1. What is the activation energy for the y -> x reaction?
Answers
Answer:
?
Explanation:
I'm very sorry I couldn't get you a answer, I haven't learned this in school.
At least not this stuff, my apologies!
HELP!!!!!!!!!
Allison and Robert have a search warrant for a suspect’s home. They know that if they announce themselves at the suspect’s home, the suspect can quickly destroy evidence. What type of warrant should Allison and Robert request?
Normally, search and seizure rules require the
rule. This means that officers must wait a reasonable amount of time for the occupant to answer. However, based on the situation, Allison and Robert request a(n)
warrant, allowing them to enter the premises immediately.
Answers
Answer:
knock and announce rule, no-knock
Explanation:
What effect does light have on the growth of plants?
Select all that apply.
Light does not have any effect on a plant’s growth.
Light makes a plant’s stem grow shorter.
Light makes a plant’s leaves turn brown.
Light will make a plant’s leaves grow bigger.
Answers
Answer:
The answer is the last one only.
Explanation:
Light/sun gives energy and growth which makes the plants leaves/plant grow bigger.
Explanation:
Without the sun, plants wouldn't get the food that's needed to grow, reproduce and survive. plants need sunlight, water and carbon dioxide to live.
What mass of precipitate (in g) is formed when 45.5 mL of 0.300 M Na3PO4 react with 42.5mL of 0.200 M Cr(NO3)3 in the following chemical reaction?
Na3PO4(aq)+ Cr(NO3)3(aq) -> CrPO4(s) +3NaNO3(aq)
Answers
The mass of the precipitate, CrPO₄ formed when 45.5 mL of 0.300 M Na₃PO₄ react with 42.5mL of 0.200 M Cr(NO3)₃ is 1.25 g
We'll begin by calculating the number of mole of Na₃PO₄ and Cr(NO3)₃ present in the solution.
For Na₃PO₄Molarity of Na₃PO₄ = 0.3 M
Volume = 45.5 mL = 45.5 / 1000 = 0.0455 L
Mole of Na₃PO₄ =?Mole = Molarity × Volume
Mole of Na₃PO₄ = 0.3 × 0.0455
Mole of Na₃PO₄ = 0.01365 mole For Cr(NO3)₃Molarity of Cr(NO3)₃ = 0.2 M
Volume = 42.5 mL = 42.5 / 1000 = 0.0425 L
Mole of Cr(NO3)₃ =?Mole = Molarity × Volume
Mole of Cr(NO3)₃ = 0.2 × 0.0425
Mole of Cr(NO3)₃ = 0.0085 moleNext, we shall determine the limiting reactant.
Na₃PO₄(aq) + Cr(NO₃)₃(aq) —> CrPO₄(s) + 3NaNO₃(aq)
1 mole : 1 mole
0.01365 : 0.0085 mole
Thus, Cr(NO₃)₃ is the limiting reactant.
Next, we shall determine the number of mole of CrPO₄ produced from the reaction.
From the balanced equation above,
1 mole of Cr(NO₃)₃ reacted to produce 1 mole of CrPO₄.
Therefore,
0.0085 mole of Cr(NO₃)₃ will also react to produce 0.0085 mole of CrPO₄.
Finally, we shall determine the mass of 0.0085 mole of CrPO₄.
Mole of CrPO₄ = 0.0085 mole
Molar mass of CrPO₄ = 52 + 31 + (16×4)
= 52 + 31 + 64
= 147 g/mol
Mass of CrPO₄ =?Mass = mole × molar mass
Mass of CrPO₄ = 0.0085 × 147
Mass of CrPO₄ = 1.25 gTherefore, the mass of the precipitate, CrPO₄ formed is 1.25 g
Learn more: https://brainly.com/question/9945440
Question is in picture below!

Answers
The enthalpy change of reaction A is -100 kJ/mol while its activation energy is 150 kJ/mol. The enthalpy for reaction B is 25kJ/mol while the activation energy is 100 kJ/mol.
What is the enthalpy change?The term enthalpy change has to do with the difference between the energy of the products and that of the reactants. We know enthalpy to be a state function so it depends on the initial and the final states of the system as it were.
The activation energy is the energy that must be possessed by the reactants as they are converted into products. It is the energy barrier that stands in between the reactants and the products in a reaction.
For reaction A;
Enthalpy = Energy of products -- Energy of reactants
= 25 kJ/mol - 125 kJ/mol = -100 kJ/mol
The activation energy is 150 kJ/mol
For reaction B;
Enthalpy = Energy of products -- Energy of reactants
= 50 kJ/mol - 25 kJ/mol = 25kJ/mol
The activation energy is 100 kJ/mol
Learn more about enthalpy:https://brainly.com/question/13996238
#SPJ1
a student has a 1 L solution of 2 M HCL and wants to increase the HCL concentration to 3 M
Answers
The student needs to add approximately 83.3 mL of 12 M HCl solution to the existing 1 L of 2 M HCl solution to increase the concentration to 3 M. It is important to handle concentrated acids with caution and follow proper safety procedures.
To increase the concentration of a 1 L solution of 2 M HCl to 3 M, the student needs to calculate the volume of concentrated HCl needed and add it to the existing solution. Here's how the calculation can be done:
Given:
Initial concentration of HCl solution = 2 M
Final concentration desired = 3 M
Initial volume of HCl solution = 1 L
Step 1: Calculate the moles of HCl in the initial solution.
Moles of HCl = Initial concentration × Initial volume = 2 M × 1 L = 2 moles
Step 2: Calculate the moles of HCl needed for the desired concentration.
Moles of HCl needed = Final concentration × Final volume = 3 M × 1 L = 3 moles
Step 3: Calculate the moles of HCl to be added.
Moles of HCl to be added = Moles needed - Moles present = 3 moles - 2 moles = 1 mole
Step 4: Convert the moles of HCl to the required volume of concentrated HCl.
To calculate the volume, we need to know the concentration of the concentrated HCl solution. Assuming it is 12 M, we can use the following formula:
Volume of concentrated HCl = Moles of HCl to be added / Concentration of concentrated HCl
Volume of concentrated HCl = 1 mole / 12 M = 0.0833 L or 83.3 mL
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
Noncovalent bonds are critically important to the function of biomolecules. Which of the following is/are (a) valid reason(s) for this importance? A.The effect of these weak interactions is cumulative. B.Their bond energies are ~10-100 times weaker than ordinary covalent bonds. C.Noncovalent interactions are made up of hydrogen bonding, electrostatic interactions, and dispersion forces. D.All of the listed statements are valid reasons for this importance.
Answers
The correct valid reason of noncovalent bonds important to function of biomolecules is A).The effect of these weak interactions is cumulative. So,correct option is A.
A noncovalent bond is a kind of synthetic bond, regularly between macromolecules, that doesn't include the sharing of sets of electrons, yet rather includes more scattered varieties of electromagnetic communications. The noncovalent bond is the predominant kind of connection between supermolecules in supermolecular chemistry.[1] Noncovalent bonds are basic in keeping up with the three-layered construction of huge atoms, like proteins and nucleic acids, and are associated with numerous natural cycles where enormous particles dilemma explicitly however fleetingly to each other.
The energy delivered in the development of noncovalent securities is on the request for 1-5 kcal per mol.[2] There are four primary kinds of non-covalent securities: hydrogen holding, ionic collaborations, Van der Waals communications, and hydrophobic bonds.[2] Instances of noncovalent securities include: those limiting connections which hold the two strands DNA in the DNA twofold helix together, those which overlap polypeptides into such optional designs as the alpha helix and the beta conformity, those which empower compounds to tie to their substrate, and those which empower antibodies to tie to their antigen.
Hence,correct option is A.
To know more about noncovalent bonds,visit here:
https://brainly.com/question/14484157
#SPJ4
What is the energy of an electron in n3 within the hydrogen atom?
Answers
The energy of an electron in the n=3 energy state of a hydrogen atom is 2.42 x 10 to the power of 19 J
Balance the following equations: 1. Mg(OH)2 + H3AsO4 → Mg3(AsO4)2 + H2O 2. NH4NO3 → N2O + H2O 3. AsCl3 + H2O → H3AsO3 + HCl 4. C4H10O2 + O2 → CO2 + H2O Use the equation for questions 5- 8: Cl2 + F2 → ClF3, 5. How many moles of Cl2 are needed to react with 3.44 moles of F2? 6. How many grams of ClF3 form when 0.204 moles of F2 react with excess Cl2? 7. How many grams of ClF3 form from 130.0 grams of Cl2 when F2 is in excess?
Answers
Answer:
A. The balanced chemical equations of the given reactions are shown below:
1. 3Mg(OH)₂ + 2H₃AsO₄ → Mg₃(AsO₄)₂ + 6H₂O
2. NH₄NO₃ → N₂O + 2H₂O
3. AsCl₃ + 3H₂O → H₃AsO₃ + 3HCl
4. 2C₄H₁₀O₂ + 11O₂ → 8CO₂ + 10H₂O
B. Number of moles of Cl₂ needed to react with 3.44 moles of F₂ = 1.14 moles
C. mass of ClF₃ produced = 0.136 * 92.5 = 12.58 g
D. 130.0 g of Cl₂ will produce (130 * 92.5)/71 g of ClF₃ = 169.37 g of ClF₃
Explanation:
A balanced chemical equation occurs when the number of the atoms involved in the reactants side is equal to the number of atoms in the products side.This in accordance with the law of conservation of mass which explains that when a chemical reaction occurs, the mass of the products should be equal to the mass of the reactants.
The balanced chemical equations of the given reactions are shown below:
1. 3Mg(OH)₂ + 2H₃AsO₄ → Mg₃(AsO₄)₂ + 6H₂O
2. NH₄NO₃ → N₂O + 2H₂O
3. AsCl₃ + 3H₂O → H₃AsO₃ + 3HCl
4. 2C₄H₁₀O₂ + 11O₂ → 8CO₂ + 10H₂O
B. The balanced equation of the reaction is: Cl₂ + 3F₂ → 2ClF₃
From the equation above, I mole of Cl₂ reacts with 3 moles of F₂
Number of moles of Cl₂ needed to react with 3.44 moles of F₂ will be = 1 * 3.44/3 = 1.14 moles
C. From the equation above, 3 moles of F₂ reacts to produce 2 moles of ClF₃
0.204 moles of F₂ will produce (2 * 0.204)/3 of ClF₃ = 0.136 moles of ClF₃
Molar mass of ClF₃ = 35.5 + 3 * 19 = 92.5 g/mol
mass of ClF₃ produced = number of moles * molar mass
mass of ClF₃ produced = 0.136 * 92.5 = 12.58 g
D. From the equation of reaction, 1 mole of Cl₂ produces 2 moles of ClF₃
molar mass of Cl₂ = 71 g/mol
71 g of Cl₂ produces 2 * 92.5 g of ClF₃
130.0 g of Cl₂ will produce (130 * 92.5)/71 g of ClF₃ = 169.37 g of ClF₃
1. Why do some combinations of ionic compounds form a precipitate while others do not?
2. Solutions of lead(II) nitrate and potassium iodide were combined in a test tube. The results of this reaction are shown below.
a. Write a formula equation for the reaction.
b. Which of the possible products is the precipitate, and how do you know?
c. Write a complete ionic equation for the reaction and identify the spectator ions.
d. Write a net ionic equation for the reaction between lead(II) nitrate and potassium iodide.
Answers
Answer:
1. Some combination of ions form a solid precipitate because it is not favorable for the ions to become solvated (dissolved). Large and lowly charged ions tend to form precipitates, especially metals such as lead, barium, and silver.
2.
a. Pb(NO3)2 + 2KI -> 2KNO3 + PbI2
b. PbI2 is a precipitate because no other combinations of cations and anions will make an insoluble compound. KI, KNO3, and Pb(NO3)2 are all soluble.
c.
\(Pb^{2+}(aq) + 2NO_3^{-}(aq) + 2K^+(aq) + 2I^-{aq} = > PbI_2(s) + 2NO_3^{-}(aq) + 2K^+{(aq)}\\\\\)
is the ionic equation. Spectator ions are NO3- and K+
d.
\(Pb^{2+}(aq)+ 2I^-{aq} = > PbI_2(s) \\\) is the net ionic equation
ask questions in comments if you have any
Mescaline a hallucinogenic amine obtained from the peyote cactus has been synthesized in two steps from 3 4 5 trimethoxybenzyl bromide The first step is nucleophile substitution by sodium cyanide. The second step is a lithium aluminum anhydride reduction. Indicate the reactions and give the structure of mescaline
Answers
Mescaline produces a wide range of psychoactive effects when ingested, including altered perception of reality, hallucinations, and euphoria. It is a powerful psychedelic drug that has been used for centuries by Native American tribes in spiritual ceremonies
Mescaline is a hallucinogenic alkaloid that is derived from the Peyote cactus. Mescaline is a complex organic molecule that can be synthesized in the laboratory from 3,4,5-trimethoxybenzyl bromide in two steps.The first step involves nucleophilic substitution using sodium cyanide, and the second step is a reduction using lithium aluminum hydride (LAH).Here's how mescaline can be synthesized from 3,4,5-trimethoxybenzyl bromide:Step 1: Nucleophilic substitution using sodium cyanideThe reaction of 3,4,5-trimethoxybenzyl bromide with sodium cyanide results in the formation of the nitrile derivative. NaCN serves as the nucleophile in this reaction, and it replaces the bromide ion.The mechanism for this reaction involves the following steps: A nucleophilic attack by the cyanide ion on the benzyl bromide. The carbon-bromine bond breaks, and the benzyl cation is formed. A second nucleophilic attack by the cyanide ion occurs on the benzyl cation, resulting in the formation of the nitrile derivative.Here's the reaction equation for this step:Step 2: Reduction using lithium aluminum hydrideThe next step is the reduction of the nitrile derivative using LAH. LAH serves as a strong reducing agent in this reaction and reduces the nitrile derivative to the amine. The mechanism for this reaction involves the following steps: A nucleophilic attack by LAH on the nitrile derivative. This results in the formation of an imine intermediate. The imine intermediate reacts with another LAH molecule, resulting in the formation of the amine.Here's the reaction equation for this step:Mescaline structure: Mescaline is a psychoactive compound that belongs to the phenethylamine class of alkaloids. The structure of mescaline is as follows: The molecule has three methoxy groups attached to the benzene ring, and it has an amine functional group. The molecule is a white crystalline powder that is soluble in water and alcohol.
for such more questions on psychoactive
https://brainly.com/question/30551262
#SPJ8
Perform the calculation and record the answer with the correct number of sig figs
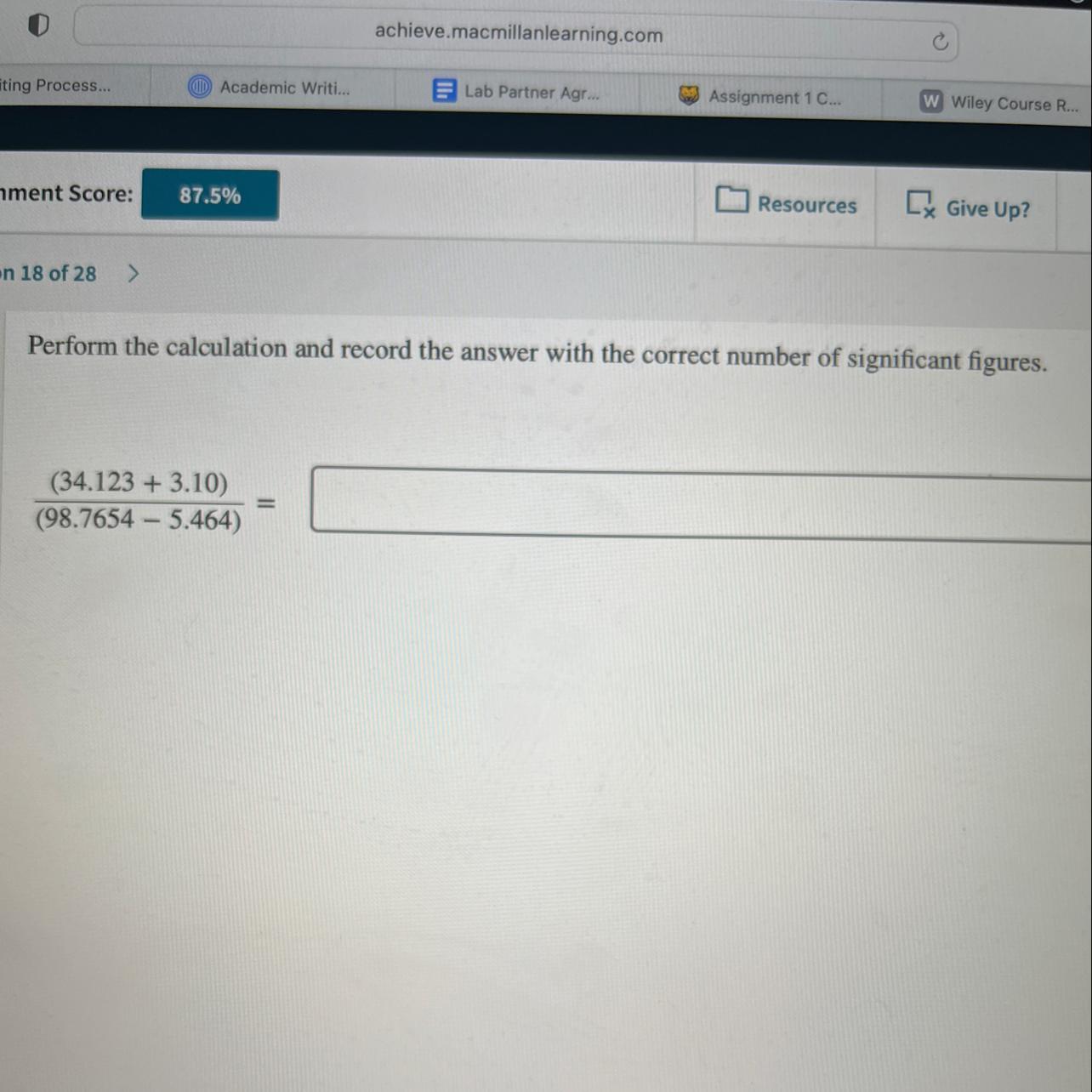
Answers
Answer:
0.399
Explanation:
Sig Fig rules
WOULD YOU BE ABLE TO FORM RUST WITHOUT OXYGEN? Explain.
Answers
Answer:
No
Explanation:
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture.
how to the chemical properties of sodium oxide allow them to be used
Answers
Answer:
Explanation:
Sodium oxide is an inorganic compound that has sodium and oxygen as its constituent
Majorly, its applications include in the ceramic industry and also aerounautical applications (for making light weight parts of air crafts)
So what exact properties of this oxide conform these properties?
The property here is that it is insoluble in water and other aqueous solutions
Furthermore, due to its ionic conductivity property, it finds use in applications like in the making of fuel cells.
How much energy is required to lower the temperature of 32.45 grams of water by 4.05 oC?
Answers
According to the specific heat capacity, 37752.33 J of energy is required to lower temperature of water.
What is specific heat capacity?Specific heat capacity is defined as the amount of energy required to raise the temperature of one gram of substance by one degree Celsius. It has units of calories or joules per gram per degree Celsius.
It varies with temperature and is different for each state of matter. Water in the liquid form has the highest specific heat capacity among all common substances .Specific heat capacity of a substance is infinite as it undergoes phase transition ,it is highest for gases and can rise if the gas is allowed to expand.
It is given by the formula ,
Q=mcΔT
It is calculated as, Q=32.45×4.2×277=37752.33 J.
Thus, 37752.33 J of energy is required to lower temperature of water.
Learn more about specific heat capacity,here:
https://brainly.com/question/2530523
#SPJ1
40ml of 0.15 molar solution of ammonia titrated with 0.15 molar of hcl. what's the ph if halfway to the quivalence point, 30ml of strong titrant is adde
Answers
The pH at halfway to the equivalence point is 2.31.
The reaction between ammonia and hydrochloric acid is; NH₃ + HCl → NH₄Cl
At halfway to the equivalence point, 30 ml of the HCl solution has been added. This means that 30/40 = 0.75 of the ammonia has reacted, leaving 0.25 of the ammonia and all of the ammonium chloride in the solution.
To calculate the pH at this point, we need to consider the equilibrium between ammonia and ammonium ions; NH₃ + H₂O ↔ NH₄⁺ + OH⁻
The equilibrium constant for this reaction is Kb = [NH₄⁺][OH⁻]/[NH₃]. At equilibrium, the concentrations of NH₄⁺ and OH⁻ are equal, so we can write Kb = [NH₃]/[NH₄⁺].
At halfway to the equivalence point, the concentration of NH₃ is 0.15 M x 0.25 = 0.0375 M, and the concentration of NH₄⁺ is also 0.0375 M (since the solution is halfway to the equivalence point). Therefore, Kb = 0.0375²/[NH₃] = 1.76 x 10⁻⁵.
Since Kb and Kw are related by the equation Kb x Kw = Ka, we can calculate Ka as: Ka = Kb x Kw
= 1.76 x 10⁻⁵ x 1 x 10⁻¹⁴
= 1.76 x 10⁻¹⁹
Now we can use the expression for the ion product of water to find the concentration of hydroxide ions:
Kw = [H⁺][OH⁻] = 1 x 10⁻¹⁴
[OH⁻] = Kw/[H⁺] = 1 x 10⁻¹⁴/[H⁺]
Since the concentration of ammonium ions is equal to the concentration of hydroxide ions at halfway to the equivalence point, we can write:
[NH₄⁺] = [OH⁻] = 1 x 10⁻¹⁴/[H⁺]
Using the equilibrium constant expression for the ionization of water, we can substitute [OH⁻] and [NH₄⁺] in terms of [H⁺]:
Ka = [NH₃][H⁺]/[NH₄⁺]
1.76 x 10⁻¹⁹ = 0.0375 x [H⁺]/(1 x 10⁻¹⁴/[H⁺])
[H⁺]² = 2.36 x 10⁻⁶
[H⁺] = 4.86 x 10⁻³ M
Finally, we can calculate the pH:
pH = -log[H⁺] = -log(4.86 x 10⁻³)
= 2.31
To know more about equivalence point here
https://brainly.com/question/4518249
#SPJ4
--The given question is incomplete, the complete question is
"40ml of 0.15 molar solution of ammonia titrated with 0.15 molar of HCl. what's the pH if halfway to the equivalence point, 30ml of strong titrant is added."--
Why is the chemical formula Li2H incorrect? Select the correct answer below: A. There should be one lithium, not two. B. There should be one lithium and two hydrogens. C. Lithium does not react with hydrogen. D. There should be three lithiums, not two.
Answers
Answer:
There should be one lithium, not two.
Explanation:
Lithium reacts with hydrogen at about 750°C to yield lithium hydride (LiH). LiH is white and powdery in appearance. It releases hydrogen gas when it reacts with water.
The correct formula for Lithium hydride is LiH and not Li2H because both lithium and hydrogen are univalent. Lithium has a valency of +1 while hydrogen has a valency of -1 in lithium hydride. Hydrides are formed between hydrogen and highly electro positive metals. In hydrides, hydrogen is forced to accept an electron from the highly electro positive metal.
How does heat travel through metals
Answers
hope this helps!!
1. Write the IUPAC names for the following 1.1 1.2 N 1.3 O NO2 x Y ·0 OH 5
Answers
1. The IUPAC name of N is nitrogen.
2. Nitrogen dioxide
3.The IUPAC name of O is oxygen
4.The IUPAC name of OH is hydroxyl.
The IUPAC name of ·0 is a radical. It is commonly found in organic chemistry and plays an important role in many reactions.
IUPAC names for the given compounds are:1.1. N: Nitrogen
The IUPAC name of N is nitrogen.
It is a non-metal and belongs to group 15 in the periodic table. It has an electronic configuration of 1s2 2s2 2p3.1.2. NO2: Nitrogen dioxide
Explanation: NO2 is a chemical compound that is formed by the combination of nitrogen and oxygen. It is a reddish-brown gas that has a pungent odor.
The IUPAC name of NO2 is nitrogen dioxide.1.3. O: Oxygen
Explanation: The IUPAC name of O is oxygen.
It is a non-metal and belongs to group 16 in the periodic table. It has an electronic configuration of 1s2 2s2 2p4.
X: UnknownExplanation: No IUPAC name can be given to an unknown compound as the structure and composition are not known.
Y: Hydroxyl Explanation: The IUPAC name of OH is hydroxyl.
It is a functional group that is composed of an oxygen atom and a hydrogen atom (-OH). It is commonly found in alcohols and phenols. ·0: RadicalExplanation: A radical is a molecule or an ion that contains an unpaired electron.
for more question on electronic configuration
https://brainly.com/question/26084288
#SPJ8
Note: The complete question is given below
Provide the IUPAC names for the following compounds:
\(CH_3CH_2CH(CH_3)CH_2CH_2CH_2CH_3\)
C6H5CH(CH3)2
H2NCH2CH2CH2CH2CH2NH2
CH3CH2CH2CH2CH2OH
CH3CH2CH2CHOHCH3
A mixture of Xe, Kr and Ar has a total pressure of 6.70 atm. What is the mole fraction of Kr if the partial pressure of Xe is 1.60 atm and that of Ar is 2.80 atm.
Answers
Answer:
0.343
Explanation:
Step 1: Given data
Total pressure of the gaseous mixture (P): 6.70 atmPartial pressure of Xe (pXe): 1.60 atmPartial pressure of Ar (pAr): 2.80 atmStep 2: Calculate the partial pressure of Kr
The total pressure of the mixture is equal to the sum of the partial pressures of the individual gases.
P = pXe + pAr + pKr
pKr = P - pXe - pAr
pKr = 6.70 atm - 1.60 atm - 2.80 atm
pKr = 2.30 atm
Step 3: Calculate the mole fraction of Kr
We will use the following expression.
X(Kr) = pKr/P
X(Kr) = 2.30 atm/6.70 atm
X(Kr) = 0.343
Considering the Dalton's partial pressure, the mole fraction of Kr is 0.34.
The pressure exerted by a particular gas in a mixture is known as its partial pressure.
So, Dalton's law states that the total pressure of a gas mixture is equal to the sum of the pressures that each gas would exert if it were alone:
\(P_{T} =P_{1} +P_{2} +...+P_{n}\) where n is the number of gases.
This relationship is due to the assumption that there are no attractive forces between the gases.
Dalton's partial pressure law can also be expressed in terms of the mole fraction of the gas in the mixture.
So in a mixture of two or more gases, the partial pressure of gas A can be expressed as:
\(P_{A} =x_{A} P_{T}\)
In this case, you know:
Total pressure= 6.70 atm. Partial pressure of Xe is 1.60 atm. Partial pressure of Ar is 2.80 atm.So, replacing in the Dalton's partial pressure law:
\(P_{T} =P_{Xe} +P_{Ar} +P_{Kr}\)
6.70 atm= 1.60 atm + 2.80 atm + \(P_{Kr}\)
6.70 atm- 1.60 atm- 2.80 atm= \(P_{Kr}\)
2.30 atm=\(P_{Kr}\)
Then: \(P_{Kr} =x_{Kr} P_{T}\)
2.30 atm= \(x_{Kr}\) 6.70 atm
\(x_{Kr}\) =2.30 atm ÷6.70 atm
\(x_{Kr}\) = 0.34
In summary, the mole fraction of Kr is 0.34.
Learn more:
brainly.com/question/14239096?referrer=searchResults brainly.com/question/25181467?referrer=searchResults brainly.com/question/1411941730 example of redox reaction
Answers
1. Combustion of gasoline in a car engine
2. Rusting of iron
3. Photosynthesis in plants
4. Respiration in animals
5. Corrosion of metals
6. Bleaching of hair with hydrogen peroxide
7. Formation of ozone in the atmosphere
8. Electroplating of metals
9. Burning of wood
10. Reaction between bleach and ammonia
11. Reaction between copper and nitric acid
12. Reaction between iron and hydrochloric acid
13. Reaction between zinc and sulfuric acid
14. Reaction between magnesium and hydrochloric acid
15. Reaction between aluminum and hydrochloric acid
16. Reaction between sodium and water
17. Reaction between potassium and water
18. Reaction between lithium and water
19. Reaction between calcium and water
20. Reaction between barium and water
21. Reaction between copper and silver nitrate
22. Reaction between lead and silver nitrate
23. Reaction between zinc and copper sulfate
24. Reaction between iron and copper sulfate
25. Reaction between magnesium and copper sulfate
26. Reaction between aluminum and copper sulfate
27. Reaction between sodium and chlorine
28. Reaction between magnesium and chlorine
29. Reaction between aluminum and chlorine
30. Reaction between zinc and hydrochloric acid.
Draw 4 decyne structure
Answers
The structure of the alkyne 4 - decyne is shown in the image attached.
How do you draw 4-decyne?Alkyne functional group compounds with a triple bond made of carbon and carbon contain 4-decyne (C). The triple bond is found at the fourth carbon position in the ten-carbon chain (decyne) of 4-decyne.
Nine hydrogen atoms (H) are joined to the first nine carbon atoms (C), starting from the left side.
In conclusion, 4-decyne is made up of a chain of ten carbon atoms, with the fourth carbon atom serving as the center of a carbon-carbon triple bond (C). Except for the carbon involved in the triple bond, all carbon atoms are connected to hydrogen atoms.
Learn more about chemical structure:https://brainly.com/question/32300619
#SPJ1

This is an riddle.
I’m lighter than what I am made of and more of me is hidden than is seen. What am I?
Whoever answers correctly gets brainly.
Answers
Answer: The answer is Iceberg
Answer:
Is it an iceberg?
Explanation:
it floats in water
and you can only see the tip of an iceberg
Hope this helps! :)
Have a great weekend!!
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Chemistry
H
D
G
A
S
F
F
F
G
Vv
F
F
F
F
F

Answers
Answer:
i think its f because my teacher have been talking about this
Explanation:
Answer:
f
Explanation: