Answers
Low-molecular-weight siderophores chelate iron with a very strong and focused affinity.
What is meant by chelate ?
It is a form of molecular bonding in chemistry wherein an ion or a molecule bind to a central metal ion.
To chelate iron from the extracellular environment, many bacteria, both G+ and G, produce and secrete siderophores. Through membrane receptors, siderophore-iron complexes are delivered into the cell.The periplasm can be reached via the iron-siderophore uptake systems because the outer membrane is not normally very permeable. Therefore, it is not surprising that bacteria have developed strategies to take advantage of these systems to deliver toxic substances that prevent the growth of competing species.
To learn more about siderophore click here:
https://brainly.com/question/11087982
#SPJ4
Related Questions
The cooling curve for a pure substance as it changes from a liquid to a solid is shows above. The solid and the liquid coexist at
Answers
The solid and the liquid coexist at the melting point, which is the point where the temperature remains constant during the phase transition.
What point does solid and liquid coexist?In the given cooling curve, the melting point is at 50°C, indicated by the flat region on the curve between points C and D. At this point, the energy being released during cooling is used to overcome the energy required for the substance to transition from a liquid to a solid state.
Once all of the substance has solidified, the temperature begins to decrease again.
Learn more about cooling curve:https://brainly.com/question/31069932
#SPJ1
Answer: all points on the curve between Q and S
Explanation: Where the graph looks like <-------->horizontal.
How many grams of NaCl

Answers
You would recover 36.525g of NaCl after evaporating all of the water.
How to find the how many grams of NaCl that would be recover when all water is evaporated off of this solution?To find the grams of NaCl that would be recovered after evaporating all the water, we can use the following formula:
mass = moles * molar mass
Where:
Moles = Molarity * Volume
Molarity = 0.250 M
Volume = 2500.0 mL = 2.5 L
Molar mass of NaCl = 58.44 g/mol
mass = 0.250 M * 2.5 L * 58.44 g/mol
mass = 36.525 g
Learn about evaporation here https://brainly.com/question/2013258
#SPJ1
In order to determine the magnetic properties of phosphorus atom we need to find out the number of unpaired electrons present in the ground state of phosphorus atom! Select one of the answers below that represents this number.
Group of answer choices
1
4
3
0
2
Answers
Answer:
Think of it this way If you have a phosphorus atom whats its oposites once you found that out you may be able to find the answer
Explanation:
Hope this helps :)
The density of a cube is 2.0gcm3. If the mass is 128 g, what is the length of each side of the cube?
Report your answer with two significant figures.
Answers
Taking into account the definition of density, the length of each side of the cube is 4 cm.
Definition of densityIn other words, density is a quantity that allows to measure the amount of mass in a certain volume of a substance.
The expression for the calculation of density is:
density= mass÷ volume
From this expression it can be deduced that density is inversely proportional to volume: the smaller the volume occupied by a given mass, the higher the density.
Length of each side of the cubeIn this case, you know that:
Density= 2 g/cm³Mass= 128 gVolume= ?Replacing in the definition of density:
2 g/cm³= 128 g÷ volume
Solving:
2 g/cm³× volume= 128 g
volume= 128 g÷ 2 g/cm³
volume= 64 cm³
The volume of a cube is equal to the measure of its side to the cube. That is to say:
Volume = side x side x side = side³
So, the length of each side of the cube can be calculated as:
64 cm³= side³
∛64 cm³= side
4 cm= side
In summary, each side of the cube has a measure of 4 cm.
Learn more about density:
brainly.com/question/14401256
brainly.com/question/952755
brainly.com/question/1462554
#SPJ1
What is the mass of 6.02 x 1024 molecules of the compound HCl?
Answers
Answer:
First, we need to determine the molar mass of HCl.
The molar mass of HCl = the mass of hydrogen (1.008 g/mol) + the mass of chlorine (35.45 g/mol) = 36.45 g/mol.
Next, we can use Avogadro's number (6.02 x 10^23 molecules/mol) to convert the number of molecules to moles:
6.02 x 10^24 molecules / 6.02 x 10^23 molecules/mol = 10 moles
Finally, we can use the molar mass to convert moles to grams:
10 moles x 36.45 g/mol = 364.5 grams
Therefore, the mass of 6.02 x 10^24 molecules of HCl is 364.5 grams.
:. It means 1 mole of Hcl
:. To find the mass of HcL
no of moles = mass/ molar mass
To get the molar mass of HCL {H=1 CL=35.5}
:. H+CL = 1+ 35.5 =36.5
So we have our molar mass and number of moles now
Then we input it in the eqn
1=x/36.5
X= 36.5g of HCl
how does ease of ion pair formation depend on concentration.
Answers
How many moles of O2 are needed to combine with 6.2 moles of phosphorus
Answers
The number of moles of O₂ are needed to combine with 6.2 moles of phosphorus is 7.75 moles.
How to calculate number of moles?The number of moles of a substance can be calculated using stoichiometry. Stoichiometry is the quantitative relationship between the reactants and products of a specific reaction or equation.
According to this question, phosphorus reacts with oxygen gas as follows:
4P + 5O₂ → 2P₂O5
Based on the above equation, 4 moles of phosphorus reacts with 5 moles of oxygen.
If 6.2 moles of P reacts, 7.75 moles of oxygen gas will be produced.
Learn more about stoichiometry at: https://brainly.com/question/30215297
#SPJ1
The table below shows the dimensions of two colored cubes.
Dimensions of Cubes
Cube Side (cm) Mass
(g)
Blue 5 250
White 4 320
Which cube is denser?
Blue, because the ratio of its mass and side is higher.
White, because the ratio of its mass and side is lower.
Blue, because it has more volume and less amount of matter.
White, because it has less volume, but a lot more matter.
Answers
i think it's d white one cause it's has less volume but more matter making its mass volume ratio high than that of the blue one
The white cube is denser, because it has less volume, but a lot more matter. So option D is correct.
What is matter ?A material called matter is made up of several kinds of particles, occupies space, and has inertia. The many sorts of particles each have a unique mass and size according to the fundamentals of current physics. The electron, proton, and neutron are three examples of material particles that are most well-known.
A material with a given mass and a certain volume in space is referred to as a matter. Examples of matters are pens, pencils, toothbrushes, water, and milk, as well as vehicles like cars, buses, and bicycles.
According to its physical and chemical makeup, "matter" can be defined as being composed of atoms. Ordinary matter is another name for such atomic stuff. For instance, since DNA molecules are composed of atoms, they fall under the notion of matter.
Thus, option D is correct.
To learn more about matter, follow the link;
https://brainly.com/question/3764450
#SPJ2
You want to know the concentration of 50.0ml of a solution of H2SO4.the endingpoint was reached when 40.0ml of 0.20M Ba(OH)2 titrant was added. Fund the concentration of the H2SO4-.
Answers
The concentration of the H2SO4 solution is 0.080 M.
The concentration of 50.0 ml of a solution of H2SO4 can be found by titrating the acid with a standardized solution of Ba(OH)2. This can be achieved by first preparing a 0.20M solution of Ba(OH)2 by dissolving a known mass of the reagent in distilled water and diluting to the mark. The end point is reached when all the H2SO4 has reacted with Ba(OH)2 and no more acid is left to react with the reagent.When 40.0 ml of 0.20M Ba(OH)2 titrant was added, we can calculate the amount of moles of Ba(OH)2 that reacted with the H2SO4 and use this value to find the concentration of H2SO4. To do this, we can use the following balanced equation:H2SO4 + 2Ba(OH)2 → BaSO4 + 2H2OFrom the balanced equation, we can see that one mole of H2SO4 reacts with two moles of Ba(OH)2. Thus, the number of moles of Ba(OH)2 used in the titration is given by:(40.0/1000) L × (0.20 mol/L) = 0.008 molWe can use the number of moles of Ba(OH)2 used to calculate the number of moles of H2SO4 present in the original solution. Since one mole of H2SO4 reacts with two moles of Ba(OH)2, the number of moles of H2SO4 in the solution is given by:0.008 mol Ba(OH)2 × (1 mol H2SO4 / 2 mol Ba(OH)2) = 0.004 mol H2SO4.The concentration of H2SO4 is then given by dividing the number of moles of H2SO4 by the volume of the solution in liters:0.004 mol / (50.0/1000) L = 0.080 M.
for such more questions on solution
https://brainly.com/question/25326161
#SPJ8
Help me please.
How do animals see their pray without light?
Answers
Answer:Many nocturnal animals have a mirror-like layer, called the tapetum, behind the retina, which helps them make the most of small amounts of light.
Explanation:
What is the formula mass of (NH4)3 PO4? (Show work)
Answers
Answer:
Mass = 149g
Explanation:
N3 = 3x14u = 42
H12 = 12x1u = 12
P = 1x31u = 31
O4 = 4x16u = 64
42+12+31+64 = 149g
871g of sodium chloride is how many moles
Answers
Answer:
14.9 mol
Explanation:
To find the number of moles in a given mass of a sample of sodium chloride (NaCl), we can multiply the number of grams in the sample by the molar mass of sodium chloride, which is 58.44 g/mol.
871 g × (1 mol / 58.44 g)
= 871/58.44 mol
≈ 14.9 mol
Note that we rounded to 3 significant figures in the final answer because that is how many significant figures were given in the mass measurement of the sodium chloride sample.
How does magnesium hydroxide provide relief for indigestion?
Answers
By interacting with hydrochloric acid to create a neutral solution, magnesium hydroxide relieves indigestion. An antacid is produced by magnesium hydroxide in the stomach.
To cure constipation and promote more bowel movements, they are only used as laxatives. Magnesium hydroxide, when present in the stomach, combines with the gastric glands' parietal cells, which produce HCl, to neutralize these acids and create a solution that is acid-free.
Hydrogen halides are substances that include hydrogen and have the chemical formula hydrochloric acid. It is an inert gas at ambient temperature, but when it comes into touch with water vapour in the air, it turns into white vapours of hydrochloric acid. Technology and industry depend on hydrogen chloride gas and hydrochloric acid.
Learn more about hydrochloric acid here
https://brainly.com/question/15102013
#SPJ4
2.0 moles of H3PO4 equals how many atoms of O
Answers
Considering the definition of compound, chemical formula and Avogadro's Number, there are 4.8184×10²⁴ atoms of O in 2 moles of H₃PO₄.
What is compound and chemical formulaIn a compound there are atoms of different elements joined by forces that we call chemical bonds.
The chemical formula indicates the number and type of different atoms present in the molecule.
Avogadro's NumberAvogadro's Number or Avogadro's Constant is called the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance. Its value is 6.023×10²³ particles per mole. Avogadro's number applies to any substance.
Atoms of OIn this case, in 1 mole of the compound H₃PO₄ you have:
3 moles of H.1 mol of P.4 moles of O.Then, in 2 moles of H₃PO₄ you have 8 moles of O.
Now, you can apply the following rule of three: if by definition of Avogadro's number 1 mole of oxygen contains 6.023×10²³ atoms, 8 moles of oxygen contains how many oxygen atoms?
\(number of atoms=\frac{8 molesx6.023x10^{23}atoms }{1 mole}\)
number of atoms= 4.8184×10²⁴ atoms
Finally, there are 4.8184×10²⁴ atoms of O in 2 moles of H₃PO₄.
Learn more about Avogadro's Number:
brainly.com/question/11907018?referrer=searchResults
brainly.com/question/1445383?referrer=searchResults
brainly.com/question/1528951?referrer=searchResults
#SPJ1
is hydrogen peroxide a product
Answers
How many carbon and hydrogen atoms are in KNO3?
Answers
Answer: 3 mol oxygen.
Explanation: Because by definition you have 1 mol of potassium nitrate. Potassium nitrate in this quantity comprises 1 mol K, 1 mol N,
PbO2 + 4HCl --- PbCl2 + Cl2 + 2H2O who buys electrons and who loses electrons?
Answers
Answer: Electrons are taken up by \(PbO_2\) and they are lost by \(HCl\)
Explanation:
Redox reaction is defined as the reaction in which oxidation and reduction take place simultaneously. It is also called the reaction where the exchange of electrons takes place.
An oxidation reaction is defined as the reaction in which a chemical species loses electrons takes place. In this reaction, the oxidation state of a substance gets increased.
A reduction reaction is defined as the reaction in which a chemical species gains electrons takes place. In this reaction, the oxidation state of a substance gets reduced.
For the given chemical reaction:
\(PbO_2+4HCl\rightarrow PbCl_2+Cl_2+2H_2O\)
The half-reactions for this redox rection follows:
Oxidation half-reaction: \(2HCl\rightarrow ClO_2 + 2e^-\)
Reduction half-reaction: \(PbO_2+2e^-\rightarrow PbCl_2\)
Hence, electrons are taken up by \(PbO_2\) and they are lost by \(HCl\)
who is sire sirol from among us?
Answers
Answer:
I'm not sure,
Explanation:
Can I have brainliest? It would help me out, if not thanks anyways! Hope this helped and have a nice day!
Why critical thinking is important especially for chemists?
Answers
Answer:
Because chemists need to think outside the box for what they do, in other words, "science is not always black and white" which means science has color to it or many different aspects some of those which are not yet discovered and needs critical thinking chemists to figure out and discover.
Explanation:
(this was all in my own words and not actually cut and Paiste ○| ̄|_ =3)
A flashbulb of volume 1.70 mL contains O2(g) at a pressure of 2.30 atm and a temperature of 29.0 °C. How many grams of O2(g) does the flashbulb contain?
Answers
The flashbulb contains approximately 0.00291 grams of O₂(g).
The ideal gas law relates the pressure (P), volume (V), temperature (T), and amount of gas (n) through the equation PV = nRT, where R is the universal gas constant. Rearranging this equation to solve for n gives n = PV/RT.
To find the amount of O₂(g) in the flashbulb, we first need to convert the volume to liters and the temperature to Kelvin:
V = 1.70 mL = 0.00170 L
T = 29.0 °C + 273.15 = 302.15 K
We can then use the ideal gas law to calculate the amount of O₂(g):
n = PV/RT
n = (2.30 atm)(0.00170 L)/(0.0821 L·atm/mol·K)(302.15 K)
n = 9.10 × 10⁻⁵ mol O₂
Finally, we can convert from moles to grams using the molar mass of O₂:
m = n × M
m = (9.10 × 10⁻⁵ mol)(32.00 g/mol)
m = 0.00291 g O₂
As a result, the flashbulb contains around 0.00291 grams of O₂(g).
To know more about the Pressure, here
https://brainly.com/question/14047938
#SPJ1
How many ml of 3M NaOH would it take to neutralize 120ml of 1M HCL?
Answers
Answer:
30ml
Explanation:
the acid-base solution just begins to turn pink and the pH reaches 7, indicating that the base has neutralized the acid. By reading the buret, it is found that 30 ml of NaOH was needed to neutralize the HCl.
Can someone help me here lol

Answers
Answer:
3) option c
4) option b
5) option a
pls mark brainliest
Draw all structural and geometric isomers of butene and name them
Answers
Alkene is an unsaturated hydrocarbon which contain at least one carbon-carbon double bond. Here butene is an alkene with the chemical formula C₄H₈. The functional group present in alkenes is the double bond.
The structural isomers are those isomers in which the atoms are completely arranged in a different order but have the same molecular formulas. It is also called the constitutional isomers.
Geometric isomers are two or more compounds with the same number and types of atoms and bonds but have different geometries for the atoms.
To know more about Geometric isomers, visit;
https://brainly.com/question/28188244
#SPJ1
करताह!
गर्मियों में घड़े का जल ठंडा क्यों होता है?
Answers
Answer:
I don't understand the question
Explanation:
what is your name
A student drew a diagram of the quantum model of an atom, as shown.
A small circle is shown. Six light gray spheres and six dark gray spheres are shown inside the circle. On the outer side of the circle is another circle of larger radius. This circle has two small circle on its boundary. Another circle of even greater radius is on the outside of the second circle. This outermost circle has four small circles on its boundary.
Which of the following explains if the student's diagram is correct or incorrect?
A - The diagram is incorrect because electrons follow an elliptical path instead of a circular path.
B - The diagram is incorrect because the exact location of the electrons cannot be determined.
C - The diagram is correct because protons and neutrons are concentrated at the center of the atom.
D - The diagram is correct because electrons are present in distinct energy levels around the nucleus.

Answers
The diagram is correct because electrons are present in distinct energy levels around the nucleus. Option D
What is the quantum model?Let nus just take a minute to be able to remind ourselves that when we look at the atom that there are several subatomic particles that can be shown to be able to make up the atom. We know that the electron is the particle that can be shown to be arranged in shells.
What we saw in the inner or the core of the atom is the nucleus and the six grey spheres that we can see there would have to do with the nucleus of the atom that is in the question.
As we now look up at the shells, we can see the electrons as they have been arranged into the respective shells were they can be found in the atom on the basis of the energy that they possess.
Learn more about atoms:https://brainly.com/question/13654549?
#SPJ1
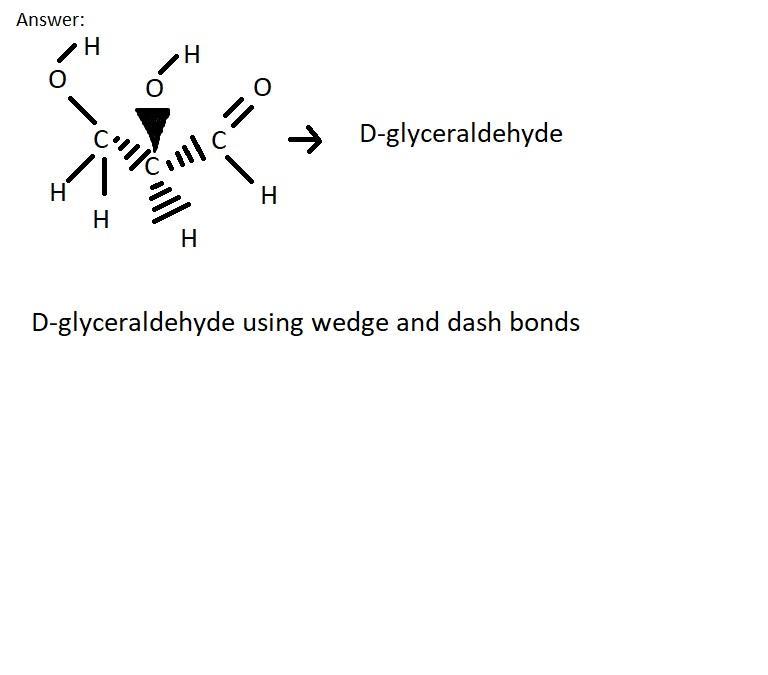
Glyceraldehyde is an aldose monosaccharide. The Fischer projection of D-glyceraldehyde is given below. Draw D-glyceraldehyde using wedge and dash bonds around the chirality center and including ALL hydrogen atoms.
Answers
Image is not given in the question, so the image for the question is given below.
Answer:
Fischer projection is a two-dimensional representation of a three-dimensional organic molecule by projection.
Wedge and dash bonds are used to represent the three-dimensional structure of a molecule, in which wedges indicates bonds towards the viewer, solid lines indicates bonds in the plane of the image and dashed lines indicates bonds away from the viewer.
Wedge and dash bonds structure of D-glyceraldehyde is attached below.

Total number of protons and neutrons in the what of an atom
Answers
Answer:
The total number of protons and neutrons is the mass number of an atom.
Explanation:
The mass number is made up of the total protons and neutrons in an atom. The mass number represents the mass of the atom in amu (atomic mass units). Electrons are not included in this representation because their mass is negligible (practically 0). The atomic number is made up of the total protons in an atom.
Which chemical formula shows an ionic compound?
Responses
NaCl
C6H12O6
N2O
CO2
Answers
Answer:
Explanation:
ionic compounds are those compounds which are held by ionic bonds which are formed by the charged cations are anions .
Here, out of the four options, Nacl is an ionic compound as na+ is a cation and cl- is an anion.
Calculate the emf of the following concentration cell: Au(s) | Au3+ (0.10 M) || Au3+ (0.50 M) | Au(s)
Answers
The emf of the cell as written is calculated to be 0.014 V.
Using the Nernst equation;
Ecell = E°cell - 0.0592/n log Q
We must note that E°cell = 0 because the anode and cathode are composed of the same type of metal.
Now;
Substituting values, we have;
Ecell = 0 - 0.0592/3 log (0.50 M)/(0.10 M)
Ecell = 0.014 V
Learn more: https://brainly.com/question/15394851
7.0 x 10 -3 mol of I2 in 100.00ml of solution
Answers
Given:
- Moles of I2: 7.0 x 10^(-3) mol
- Volume of solution: 100.00 mL (which is equal to 0.1000 L)
Molarity (M) = Moles of solute / Volume of solution in liters
Molarity = (7.0 x 10^(-3) mol) / (0.1000 L)
Molarity = 0.070 M
Therefore, the concentration of the I2 solution is 0.070 M.