Answers
According to Bohr's model of the atom, the energy of the photon involved in the transition of a hydrogen atom's electron from "n" is given by the equation:
E (n) = - \(\frac{1}{n^{2} }\) . 13.6 eV
Here, "n" represents the shell number.
Bohr's model of hydrogen puts forward the assumption that the electrons revolve around the nucleus in specific orbits or shells.
Bohr explained the concept of energy of a photon in the hydrogen spectrum in terms of absorption and emission of photons by electrons, which leads to change in energy levels. The energy of a photon is given by the equation:
ΔE =hν
where, h is planck's constant whose value is 6.626 × 10⁻³⁴ Js
and ν is the frequency of the photon.
A drawback of Bohr's model is that it doesn't work for systems consisting of more than one electron.
To know more about Bohr's model of atom here
https://brainly.com/question/24708000
#SPJ4
Related Questions
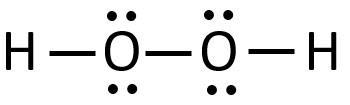
Construct a Lewis structure for hydrogen peroxide, H2O2, in which each atom achieves a stable noble-gas electron configuration. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons.
Answers
Answer:
See explanation and image attached
Explanation:
Hydrogen peroxide is made up of two atoms of hydrogen and two atoms of oxygen as shown in the image attached.
The two oxygen atoms are joined together by a single covalent bond and each of the oxygen atoms are bonded to one hydrogen atom each.
There are two lone pairs on each of the oxygen atoms.
The Lewis(dot) structure for hydrogen peroxide is shown in the image attached to this answer.
Vanessa walks 189m northward, then takes a right and walks 238m eastward. What is the magnitude of her displacement?
Answers
The magnitude of Vanessa's displacement is the square root of the sum of the squares of her northward and eastward movements:
sqrt(189^2 + 238^2) = 285.3 m.
~ happy valentine's day
6. According to the graph above, which paper towel brand adsorbed the 5 points
most liquid?
Bounty
Brawny
Viva

Answers
Use your data, the equation to the right, and the specific heat of water (4.184 J/g C) to compute the specific heat values of each metal. Use a calculator and round to the nearest hundredth place.
Answers
The heat capacity for the metals are;
Aluminum - 0.89
Copper - 0.11
Iron - 0.44
Lead - 0.12
What is the specific heat?The specific heat of a substance is denoted by the symbol "C" and is typically measured in units of J/g·°C (joules per gram per degree Celsius) or cal/g·°C (calories per gram per degree Celsius).
The specific h
We have that;
For Aluminum;
c = 4.184 * 39.85 * 4.7/11.98 * 72.9
= 783.6/873.3
= 0.89
For Copper;
c = 4.184 * 12.14 * 1.9/12.14 * 75.4
= 96.5/915.3
= 0.11
For Iron
c = 4.184 * 40.24 * 2.4/12.31 * 75.1
= 404.1/924.5
= 0.44
For Lead
c = 4.184 * 39.65 * 0.7/12.46 * 76.7
c = 116.1/955.68
= 0.12
Learn more about specific heat:https://brainly.com/question/31608647
#SPJ1

Which of these casting methods is the most popular, accounting for approx. 70% of all metal casting?
a. Sand casting
b. Shell casting
c. Investment casting
d. Lost foam casting
Answers
Sand casting, which makes up around 70% of all metal casting, is the most common casting technique. A design of the finished object is created in wood or metal and employed in the process of "sand casting,".
Casting techniques are a class of manufacturing procedures used to turn liquid materials like metal, plastic, or ceramics into solid things. The liquid substance is put into a mould and given time to harden and assume the contours of the hollow. Casting techniques come in a variety of forms, including sand casting, investment casting, die casting, shell casting, and lost foam casting. The kinds of materials that can be cast, the degree of accuracy and surface quality, and the complexity of the pieces that can be created are only a few of the distinctive characteristics that each casting technique has. The automobile, aerospace, and construction industries all use casting techniques extensively.
Learn more about casting methods here:
https://brainly.com/question/15359594
#SPJ4
How many resonance structures does the sulfur dioxide molecule have?
Answers
Sulphur dioxide molecule have two resonance structure.
Resonance structures are the different Lewis structures of the same molecule. The components of the molecule will change places from one another.
The two resonance structures of sulphur dioxide molecule is demonstrated here.
A third Lewis structure can also be drawn. But that is not a stable molecule in reality and can't be counted.

To solve this, we must be knowing each and every concept related to resonance. Therefore, there are total two resonance structure of sulfur dioxide molecule.
What is resonance?Resonance is a technique for characterizing delocalized electrons in molecule in which the bonding cannot be described directly by a singular Lewis structure. It is also called mesomerism.
Each unique Lewis structure of the targeted molecule or ion is referred to as a contributing structure. Because they simply differ in the location of delocalized electrons, contributing structures really aren't isomers of the targeted molecule or ion. There are total two resonance structure of sulfur dioxide molecule.
Therefore, there are total two resonance structure of sulfur dioxide molecule.
To know more about resonance, here:
https://brainly.com/question/11331041
#SPJ2
Gas Laws
Pre-Test Active
1
2 3
5
6
O final pressure
O atmospheric pressure
O combined pressure
O partial pressure
7 8
9
10
A scientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide,
oxygen, and nitrogen. Which term most likely describes what she is measuring?
Answers
The term that the scientist would use in this case is partial pressure. Option D
What is the partial pressure?
The pressure that one particular gas component within a mixture of gases exerts is referred to as partial pressure. It is the pressure that the gas would experience at the same temperature if it were the only thing in the entire volume.
When researching gas mixtures, such as in gas laws, gas phase equilibria, and gas collecting methods, partial pressures are extremely crucial for the gas.
Learn more about partial pressure:https://brainly.com/question/30114830
#SPJ1
3. In a chemical reaction, the final amount of the products is determined by the
A. Universal Gas Law
B. catalysts involved
C. air pressure
D. temperature
E. limiting reactant
Answers
If you run out of the limiting reactant you cannot make anymore of a product.
How long it take for skin cell to divide
Answers
Answer:
This process of growing, moving and shedding takes about four weeks. SO I would think dividing is around a month
Explanation:
Hope this helps! Consider Brainiest! <3
Answer:
1/2 to 1 and 1/2 hours
Explanation:
Skin cells go through the division phase that takes between 1/2 to 1 and 1/2 hours to complete, depending on the location. Body cells, which include skin, hair, and muscle, are duplicated through the process of mitosis. Skin cells belong in the category of somatic cells and are duplicated at a rapid rate during life.
What is the correct order for the first three steps of the scientific method?
A. State the question, conduct an experiment, form a hypothesis
B. Form a hypothesis, form a conclusion, conduct an experiment
C. Conduct an experiment, form a hypothesis, analyze the data
D. State the question, form a hypothesis, conduct an experiment
SUBMIT
Answers
Answer:
D.) State the question, form a hypothesis, conduct an experiment
Explanation:
https://www.colorincolorado.org/article/steps-scientific-process
A sample of gas has a volume of 100. L at 17 °C and 800. torr. To what temperature must the gas be cooled in order for its volume to become 50.0 L at a pressure of 600. torr? Your answer will need to be in Kelvin.
Source
StylesNormalFontSize
Answers
Answer:
108.81 K
Explanation:
First convert 17 °C to Kelvin:
17 + 273.16 = 290.16 KAssuming ideal behaviour, we can solve this problem by using the combined gas law, which states that at constant composition:
P₁V₁T₂=P₂V₂T₁Where in this case:
P₁ = 800 torrV₁ = 100 LT₂ = ?P₂ = 600 torrV₂ = 50 LT₁ = 290.16 KWe input the data:
800 torr * 100 L * T₂ = 600 torr * 50 L * 290.16 KAnd solve for T₂:
T₂ = 108.81 KWhat mass of lithium phosphate would you mass to make 2.5 liter of 1.06 M lithium
phosphate solution?
Answers
Answer:
Approximately \(3.06 \times 10^{2}\; \rm g\) (approximately \(306\; \rm g\).)
Explanation:
Calculate the quantity \(n\) of lithium phosphate in \(V = 2.5\; \rm L\) of this\(c = 1.06\; \rm M = 1.06\; \rm mol \cdot L^{-1}\) lithium phosphate solution.
\(\begin{aligned}n &= c \cdot V\\ &= 2.5\; \rm L \times 1.06\; mol \cdot L^{-1}\\ &= 2.65\; \rm mol\end{aligned}\).
Empirical formula of lithium phosphate: \(\rm Li_3PO_4\).
Look up the relative atomic mass of \(\rm Li\), \(\rm P\),and \(\rm O\) on a modern periodic table:
\(\rm Li\): \(6.94\).\(\rm P\): \(30.974\).\(\rm O\): \(15.999\).Calculate the formula mass of \(\rm Li_3PO_4\):
\(M(\rm Li_3PO_4) = 3 \times 6.94 + 30.974 + 4 \times 15.999 = 115.79\; \rm g \cdot mol^{-1}\).
Calculate the mass of that \(n = 2.65\; \rm mol\) of \(\rm Li_3PO_4\) formula units:
\(\begin{aligned}m &= n \cdot M \\ &= 2.65\; \rm mol \times 115.79\; \rm g\cdot mol^{-1} \\ &\approx 3.06 \times 10^{2}\; \rm g \end{aligned}\).
How many grams of CO2 are made from 10 moles of O2?
Answers
Answer:
100
Explanation:
A car's engine block is made of steel and has a mass of 21080g. How much heat (J) is absorbed by the engine block when its temperature is raised from 20°C to 90°C?
Answers
The heat absorbed by the engine block when its temperature is raised from 20°C to 90°C is 665,640 J.
To calculate the heat absorbed by the engine block, we can use the equation:
Q = mcΔT
where Q is the heat absorbed, m is the mass of the engine block, c is the specific heat capacity of steel, and ΔT is the change in temperature.
First, we need to calculate the specific heat capacity of steel. The specific heat capacity of steel is typically around 0.45 J/g°C.
Using this value and the given values of mass and temperature change, we can calculate the heat absorbed by the engine block as follows:
Q = (21080 g) x (0.45 J/g°C) x (90°C - 20°C)
Q = 21080 g x 0.45 J/g°C x 70°C
Q = 665,640 J
For more question on heat absorbed click on
https://brainly.com/question/30361927
#SPJ11
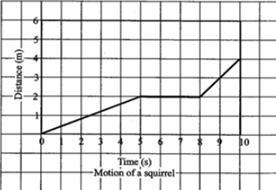
The above graph shows the journey of squirrel for a certain interval. Which of the following describes the motion of the squirrel between 5 s and 8 s?
Answers
The statement that describes the motion of the squirrel between 5s and 8s is as follows: The squirrel's speed did not change (option C).
What is a graph?A graph in mathematics and statistics is a data chart (graphical representation of data) intended to illustrate the relationship between a set (or sets) of numbers (quantities, measurements or indicative numbers) and a reference set, whose elements are indexed to those of the former set(s) and may or may not be numbers.
According to this question, a graph is used to illustrate the relationship between the distance moved by a squirrel and the time it took it to move i.e. the journey of squirrel for a certain interval or period of time.
Based on the graph above, the speed increased with time up until 5 seconds, however, from 5seconds till 8 seconds, the speed of the squirrel remained constant i.e. did not change until it picked up again.
Therefore, option C is the correct description of the situation of the squirrel between 5s to 8s.
The options to the incomplete question are as follows:
A. The squirrel's speed increased
B. The squirrel's speed decreased
C. The squirrel's speed did not change
D. The squirrel moved backward
Learn more about graph at: https://brainly.com/question/17267403
#SPJ1
Which of the following stage is also known as the unstable stage?
a.Transient creep stage
b.Constant creep stage
c.Fracture stage
d.Steady stage creep stage
Answers
Transient stage is also called the unstable stage and in this stage, there is a gradual decrease in deformation rate to a definite constant value.
What is Transient stage ?A transient state is when a process variable or variables changes, but before the system reaches a steady state.
Transient time is the time it takes for a circuit to change from one steady state to the next.
Therefore, Transient stage is also called the unstable stage and in this stage, there is a gradual decrease in deformation rate to a definite constant value.
Learn more about Transient stage here ;
https://brainly.com/question/22686897
#SPJ1
two compounds (a and b) are reacted with iodine using a process similar to the one used in this lab. use the data below to determine the order of the reaction with respect to compound a. [a](m) [b](m) [i2] (m) rate(m/s) 4.0 1.0 0.0050 0.000043 8.0 1.0 0.0050 0.00017 question 3 options: 0 2 1 3
Answers
The reaction order with respect to compound A is 2. Solution: 2.
Determine the order of the reaction with respect to compound.Let us compare reaction rates at [A] = 4.0 M and [A] = 8.0 M:
0.000043 M/s at [A] = 4.0 M
0.00017 M/s at [A] = 8.0 M
To determine the order of the reaction with respect to A, we can use the following equation:
rate = k(A)x(B)y
where k is the rate constant
x = order in relation to A
y = position in relation to B
We can simplify the equation by assuming that the order with respect to B is zero (i.e., B has no effect on the rate of the reaction).
k[A]x = rate
Taking the ratio of the two rates we calculated earlier, we get:
(0.00017 M/s) / (0.000043) = (k[8.0]x) / (k[4.0]x)
When we simplify, we get:
4 = (8^x) / (4^x)
When we take the logarithm of both sides, we get:
x log(4) = log(4) (2)
2 x = log(4) / log(2)
To Know more about Compound Visit:
brainly.com/question/14117795
#SPJ1
how do you balance this equation
2h2s+3o2+so2
Answers
The balanced equation is: 4 \(H_2S\)+ 3 \(O_2\)→ 4 \(SO_2\)+ 8 \(H_2O\)
The given chemical equation is unbalanced. To balance it, we need to adjust the coefficients in front of each chemical species until the number of atoms on both sides of the equation is equal.
The unbalanced equation is:
2 \(H_2S\)+ 3 \(O_2\)→ \(SO_2\)
Let's start by balancing the sulfur (S) atoms. We have two sulfur atoms on the left side and one sulfur atom on the right side. To balance the sulfur, we can place a coefficient of 2 in front of the \(SO_2\):
2 \(H_2S\)+ 3 \(O_2\)→ 2 \(SO_2\)
Now, let's balance the hydrogen (H) atoms. We have four hydrogen atoms on the left side (2 from each \(H_2S\)) and none on the right side. To balance the hydrogen, we can place a coefficient of 4 in front of the water (H2O) on the right side:
2 \(H_2S\)+ 3 \(O_2\)→ 2 \(SO_2\)+ 4 \(H_2O\)
Finally, let's balance the oxygen (O) atoms. We have six oxygen atoms on the right side (3 from \(O_2\) and 3 from 2 \(SO_2\)) and three on the left side (2 from \(H_2S\)). To balance the oxygen, we can place a coefficient of 3/2 in front of the O2:
2 \(H_2S\)+ (3/2) \(O_2\)→ 2 \(SO_2\)+ 4 \(H_2O\)
To remove the fractional coefficient, we can multiply all coefficients by 2:
4 \(H_2S\) + 3 \(O_2\)→ 4 \(SO_2\)+ 8 \(H_2O\)
Now the equation is balanced, with an equal number of atoms on both sides. The balanced equation is:
4 \(H_2S\)+ 3 \(O_2\)→ 4 \(SO_2\)+ 8 \(H_2O\)
For more such questions on balanced equation visit:
https://brainly.com/question/23877810
#SPJ8
a weather balloon is inflated to a volume 2.2 10square3 L with 374g of helium. what is the density of helium in grams per liter
Answers
Answer:
Density = 0.17 g/L
Explanation:
It is given that,
Volume of the inflated balloon filled with Helium, \(V=2.2\times 10^3\ L\)
Mass, m = 374 g
We need to find the density of helium. It is equal to its mass per unit volume. It can be given by :
d =m/V
\(d=\dfrac{374\ g}{2.2\times 10^3\ L}\\\\=0.17\ g/L\)
So, the density of helium in the balloon is 0.17 g/L.
CALCULATE SCIENTIFIC NOTATION (Add and Subtract)
1) 9 × 103 + 2.3 × 104
2) 15 × 102 + 5.2 × 105
3) 10 × 104 + 2.8 × 106
4) 7.0 × 103 + 8.6 × 104
5) 3.0 × 104 + 14.5 × 105
6) 8 x 104 – 2.7 x 102
7) 5.0 x 103 – 8.9 x 104
8) 7.0 x 103 – 8.20 x 102
Answers
Answer:
1. 1166.2
2. 2076
3. 1336. 8
4. 733.04
5. 1834.5
6. 556.6
7. - 410.6
8. 836.4
Explanation:
1.9 x 103 =928
1. Why do magnets repel each other?
2. Why do magnets attract some metal objects?
3. What happens if a magnet breaks in ½?
4. How do you make a piece of metal magnetic?
Please please help
Answers
family of macromolecules are composed of carbon, hydrogen and oxygen in a 1:2:1 ratio and includes sugars, starches, glycogen, and cellulose. multiple choice question. protein lipid nucleic acid carbohydrate need help? review these concept resources.
Answers
Macromolecules that are composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio and include sugars, starches, glycogen, and cellulose is carbohydrate.
Macromolecules: Carbohydrate, Lipid, Protein, and Nucleic acidMacromolecules are molecules that are made of small molecules (monomers).
There are 4 types of macromolecules: carbohydrate, lipid, protein, and nucleic acid.
Carbohydrate is made up of monomer glucose. Each compound is composed of carbon, hydrogen, and oxygen in a 1: 2: 1 ratio. Carbohydrate is classified into polysaccharide (glycogen, starch, cellulose), disaccharide (lactose, sucrose, maltose), and monosaccharides (glucose, galactose, and fructose). Protein is composed of monomer amino acids. The elements of protein are carbon, hydrogen, oxygen, and nitrogen, also has -COOH, -NH2, and R groups.Lipids contain fatty acid and glycerol chains. The element that makes up the lipid are carbon, hydrogen, and oxygen (not in a 1: 2: 1 ratio). For example fats, and oils.Nucleic acid contains elements carbon, hydrogen, oxygen, nitrogen, and phosphorus. For example DNA, RNAThus, sugars, starches, glycogen, and cellulose are included in carbohydrates. The ratio of elements in carbohydrates is 1:2:1.
Learn more about macromolecules by clicking this link :
https://brainly.com/question/17637031
#SPJ4
Experiment 4: A chemist mixes aqueous solutions of sodium hydroxide and aluminum chloride in a double-displacement reaction, which forms a white solid precipitate and a clear solution. Write the complete, balanced molecular equation for the reaction. Include physical states.
balanced equation:
Answers
The balanced molecular equation for the reaction between sodium hydroxide (NaOH) and aluminum chloride (\(AlCl_3\)) in aqueous solution can be written as follows: 2NaOH(aq) + 3\(AlCl_3\)(aq) → 3NaCl(aq) + \(Al(OH)_3\)(s)
In this reaction, sodium hydroxide (NaOH) reacts with aluminum chloride (\(AlCl_3\)) to form sodium chloride (NaCl) and aluminum hydroxide (\(Al(OH)_3\)). The coefficients in the balanced equation indicate the stoichiometric ratio between the reactants and products.
The physical states of the substances are indicated by the symbols (aq) for aqueous solutions and (s) for the solid precipitate.
The reaction is a double-displacement reaction, also known as a precipitation reaction. Double-displacement reactions involve the exchange of ions between two compounds, resulting in the formation of a precipitate.
In this case, sodium hydroxide and aluminum chloride react to form sodium chloride and aluminum hydroxide, with aluminum hydroxide being the white solid precipitate.
It's worth noting that the actual reaction might involve hydrated forms of the compounds, such as NaOH·x\(H_2O\) and \(AlCl_3\)·y\(H_2O\). However, for simplicity, these hydrated forms are not included in the balanced equation.
Overall, the balanced equation represents the chemical reaction between sodium hydroxide and aluminum chloride, showing the reactants, products, and their stoichiometric ratios.
For more such question on balanced molecular equation visit:
https://brainly.com/question/11904811
#SPJ8
Why is it important that babies' skulls have soft spots (fontanels)?
Answers
Explanation:
These soft areas are the gaps between the skull's bones, when bone growth hasn't finished. As a result, the skull can be shaped during birth. By the age of two to three months, the smaller spot at the back usually closes. Around age 18 months, the larger spot in the front frequently closes.
J00
Sugar
(C2H2011)
260
KNO
220
180
Solubility (g solute per 100 g H,0)
140
NaNO,
NaBr
100
KBr
60
КСІ
Naci
20
0
0
20
Ce (50)
40 60
Temperature (°C)
80
100
Which compound would make a saturated solution if 98 grams were
dissolved in 100 grams of solution at 80 degrees Celsius?
O KBr
O Sugar
OKCI
O NaCl
alish

Answers
Salt and water
Sand and water
Flour and water
beans and water
which mixture is a suspention?How do you know this?
Answers
Answer:
Step-by-step explanation-
A suspension is a heterogeneous mixture of a fluid that contains solid particles sufficiently large for sedimentation.
Particles are big enough to be visible to the eye and eventually with time they settle to the bottom of the container. The maximum amount of solute can be separated through physical methods like filtration.
In the mentioned cases all of them except salt in water are suspensions since the particles of each of them are big enough and do not get dissolved in water easily and can be separated from the mixture.
The heat capacity of copper metal is 0.38 J/goC. Assume you had a 75 g cube of copper at 25.0oC. What would the final temperature of the copper be (in oC) if it absorbed 150 J of heat?
Answers
The final temperature of the copper would be 30.26°C if it absorbed 150 J of heat.
To solve this problem, we can use the formula:
q = m * C * deltaT
where q is the heat absorbed by the copper, m is the mass of the copper, C is the heat capacity of copper, and deltaT is the change in temperature.
Rearranging the formula, we get:
deltaT = q / (m * C)
Substituting the given values, we get:
deltaT = 150 J / (75 g * 0.38 J/g°C) = 5.26 °C
Therefore, the final temperature of the copper will be:
25.0°C + 5.26°C = 30.26 °C
Learn more about heat capacity, here:
https://brainly.com/question/28302909
#SPJ1
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
According to the law of conservation of energy, in theory a bouncy ball should not stop bouncing. But however, we know that it eventually stops. Where does the energy go? Used reasoning and evidence to explain
Answers
A bouncing ball gradually stops bouncing because its energy is converted to heat energy.
According to the law of conservation of energy, energy is neither created nor destroyed but can be transformed from one form to another.
When a ball is bouncing up and down, we notice that the ball will slow down gradually. This is because, the energy in the bouncing ball is transferred to the small air molecules inside the ball as heat. Hence, the ball looses energy consistently until it comes to a stop.
Learn more: https://brainly.com/question/1527403