Use the balanced equation to solve the problem.Zn + 2HCI ZnCl₂ + H₂2.90mol of zinc are placed in a beaker.How many moles of HCI are required to react with all the zinc?H mol
Answers
Answer
We have the following chemical reaction:
\(Zn+2HCl\rightarrow ZnCl_2+H_2\)We can see that the equation is balanced because we have the same number of each atom in both sides of the equation.
Now, as we can see in the equation, for every mol of Zn that reacts it is needed 2 moles of HCl. Therefore we can calculate the moles of HCl needed in total as follows:
\(n_{HCl}=2.9mol.\frac{2molHCl}{1molZn}=5.8mol\)So the answer is: there are needed 5.8 mol.
Related Questions
The following set of data was obtained by the method of initial rates for the reaction:S2O82-(aq) + 3 I-1(aq) → 2 SO42-(aq) + I3-1(aq)What is the initial rate when S2O82- is 0.15 M and I- is 0.15 M?Exp [S2O82-] (M) [I-1] (M) Rate (M/s)1 0.25 0.10 9.00 x 10-32 0.10 0.10 3.60 x 10-33 0.20 0.30 2.16 x 10-2Seleccione una:a. 5.40 × 10-2 M s-1b. 1.22 × 10-2 M s-1c. 4.10 × 10-6 M s-1d. 8.10 × 10-3 M s-1
Answers
The initial rate for the reaction when [S₂O₈²⁻] is 0.15 M and [I-] is 0.15 M is 8.10 × 10⁻³ M s⁻¹
How do we calculate the initial rate for the reaction?The rate law states
Rate = k(S₂O₈²⁻)(I⁻)
The rate constant, k, can be determined by substituting the rate and concentrations from any of the experiments into the rate law. Using Experiment 1, we have:
9.00 x 10⁻³M/s = k(0.25M)(0.10)
k = (9.00 x 10⁻³ M/s)/(0.25M)(0.10M)
k = 36 x 10⁻²M⁻¹S⁻¹
Rate = (3.6 x 10⁻²M⁻¹S⁻¹)(0.15M)(0.15M)
Rate = 8.10 x 10⁻³M/s
The above answer is based on the full question below
The following set of data was obtained by the method of initial rates for the reaction
S₂O₈²⁻(aq) + 3 I-1(aq) → 2 SO₄²⁻(aq) + I3-1(aq)
What is the initial rate when S2O82- is 0.15 M and I- is 0.15 M?
Exp [S₂O₈²⁻] (M) [I-1] (M) Rate (M/s)
1 0.25 0.10 9.00 x 10⁻³
2 0.10 0.10 3.60 x 10⁻³
3 0.20 0.30 2.16 x 10⁻²
Select one una:
a. 5.40 × 10-2 M s⁻¹
b. 1.22 × 10-2 M s⁻¹
c. 4.10 × 10-6 M s⁻¹
d. 8.10 × 10-3 M s⁻¹
Find more exercises on initial rate of reaction;
https://brainly.com/question/31524964
#SPJ1
Which member of the alkali metals has the highest electronegativity?
A) Rb
B) Na
C) Fr
D) Cs
E) Li
F) K
Answers
Lithium alkali metals has the highest electronegativity. An atom's ability to attract the electrons of a bond grows with electronegativity, a feature of an atom.
The electrons in a covalent bond are shared evenly by two bound atoms if their electronegativity levels are the same. Typically, the more electronegative atom attracts the electrons in a chemical bond more so than the other one. Consequently, a polar covalent bond is created. The electrons aren't shared at all if the electronegativity values are significantly dissimilar. Ionic bonds are created when one atom essentially steals the other atom's bond electrons.
Over a period, electronegativity often increases as it moves from left to right. The noble gases frequently defy this rule.
Moving down on the periodic table often results in a decrease in electronegativity.
To know more about Ionic bonds visit
https://brainly.com/question/9075398
#SPJ4
What shape is formed from water?
Linear
Bent 120
Bent 109.5
Trigonal Planar
Answers
Answer:
linear
Explanation:
.....................…...…
Explanation:
bent with an H-0-H angle if 104.5°
A student knew that it was to make copper (ii) sulphate crystals by adding copper (ii) carbonate to dilute sulphuric acid and warming the resulting solution to drive off some of the water and then leaving the solution to crystallize. he decided to make copper (ii) nitrate crystals by similar method. the only change he made was to use dilute nitric acid. he left the final solution for several days but failed to collect any crystals. explain why the student did not get any crystals to collect
Answers
The student did not get any crystals to collect because copper (II) nitrate is a soluble salt, meaning it readily dissolves in water. Unlike copper (II) sulphate, which is insoluble and can crystallize out of solution, copper (II) nitrate remains in solution even after evaporation of the water.
When the student added copper (II) carbonate to dilute nitric acid, a reaction occurred to produce copper (II) nitrate and carbon dioxide gas. However, since copper (II) nitrate is soluble, it remained dissolved in the solution. The student then warmed the solution to drive off some water, but this step did not cause the copper (II) nitrate to crystallize because it remains soluble in the remaining water.
Leaving the solution to crystallize for several days did not result in the formation of copper (II) nitrate crystals because there was no solid salt to crystallize out. The water gradually evaporated, but the dissolved copper (II) nitrate simply became more concentrated in the remaining solution.
To obtain copper (II) nitrate crystals, the student would need to use a different method. One possible approach is to use a more concentrated nitric acid solution or to try different solvents that can selectively precipitate the copper (II) nitrate from the solution. It's important to note that working with chemicals and performing experiments should always be done with proper safety precautions and under the supervision of a qualified individual.
To know more about copper (II) sulphate, refer to the link below:
https://brainly.com/question/14490609#
#SPJ11
Ammonium nitrate criss cross formula
Answers
Answer:
By using the criss cross method,the -1 charge of nitrate ion is shifted to ammonium ion and +1 charge of ammonium ion is shifted to nitrate ion. In this way, the final formula NH4NO3 is formed for the ammonium nitrate.
rank the following in order of decreasing δ and energy of light absorbed. a: [cr(en)3]3 b: [cr(cn)6]3− c: [crcl6]3−
Answers
The order of decreasing δ and energy of light absorbed for the compounds [Cr(en)3]3+, [CrCl6]3-, and [Cr(CN)6]3- is as follows: [Cr(en)3]3+ > [CrCl6]3- > [Cr(CN)6]3-.
In the given order, [Cr(en)3]3+ has the highest value of δ and absorbs light with the highest energy. This can be attributed to the presence of the ethylenediamine ligands (en), which are strong field ligands. The strong field ligands cause a larger splitting of the d-orbitals in the central chromium ion, resulting in a higher energy gap between the ground state and excited states. Therefore, [Cr(en)3]3+ exhibits a higher δ and absorbs light with higher energy.
On the other hand, [Cr(CN)6]3- has the lowest value of δ and absorbs light with the lowest energy. This is because cyanide ligands (CN) are weak field ligands, leading to a smaller splitting of the d-orbitals and a lower energy gap. As a result, [Cr(CN)6]3- has the lowest δ and absorbs light with lower energy compared to the other two compounds.
In between these, [CrCl6]3- falls in the middle with intermediate values of δ and energy of light absorbed. Chloride ligands (Cl) are moderately strong field ligands, causing a moderate splitting of the d-orbitals and an intermediate energy gap.
In summary, the order of the compounds with decreasing δ and energy of light absorbed is [Cr(en)3]3+ > [CrCl6]3- > [Cr(CN)6]3-. This order is determined by the strength of the ligands and the resulting splitting of the d-orbitals, which influences the energy gap and the energy of light absorbed by the compounds.
cyanide https://brainly.com/question/13246521
#SPJ11
A student wants to make a 0.600 M aqueous solution of barium sulfate, BaSO4, and has a bottle containing 12.00 g of barium sulfate. What should be the final volume of the solution?
Find the numerical answer for this question and make sure to include the following:
What is the formula for molarity?
What is the molar mass for barium sulfate?
When you give your numerical answer, what are the correct significant figures and how do you know that is the correct amount?
Need this ASAP!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Answers
Answer: The volume of the solution is 85.7 mL
Explanation:
Molarity is defined as the amount of solute expressed in the number of moles present per liter of solution. The units of molarity are mol/L. The formula used to calculate molarity:
\(\text{Molarity of solution}=\frac{\text{Given mass of solute}\times 1000}{\text{Molar mass of solute}\times \text{Volume of solution (mL)}}\) .....(1)
We are given:
Molarity of solution = 0.600 M
Given mass of \(BaSO_4\) = 12.00 g
We know, molar mass of \(BaSO_4=[(1\times 137.33)+(1\times 32.07)+(4\times 16)]=233.4g/mol\)
Putting values in equation 1, we get:
\(0.600=\frac{12.00\times 1000}{233.4\times \text{Volume of solution}}\\\\\text{Volume of solution}=\frac{12.00\times 1000}{233.4\times 0.600}=85.68mL=85.7mL\)
The rule of significant number that is applied for the problems having multiplication and division:
The least number of significant figures in any number of the problem determines the number of significant figures in the answer.
Here, the least number of significant figures is 3 that is determined by the number, 0.600. Thus, the answer must have these many significant figures only.
Hence, the volume of the solution is 85.7 mL
The half-life for Carbon-14 is 5614 years. An ancient piece of cloth is found to contain ¼ of its original Carbon-14. How old is the cloth? Describe or show in detail how you solved this.
Answers
Answer:
To determine the age of the ancient cloth, we can use the concept of radioactive decay and the half-life of Carbon-14.
Carbon-14 is a radioactive isotope of carbon, which decays over time into nitrogen-14 through beta decay. The half-life of Carbon-14 is 5614 years, which means that after 5614 years, half of the original amount of Carbon-14 in a sample will have decayed.
In this case, the cloth contains only ¼ of its original Carbon-14. This means that three half-lives have passed since the cloth was first created, as each half-life reduces the amount of Carbon-14 by half.
To determine the age of the cloth, we can use the following formula:
N = N0(1/2)^t/T
where N is the current amount of Carbon-14 in the cloth, N0 is the original amount of Carbon-14 in the cloth, t is the time that has passed, and T is the half-life of Carbon-14.
We know that N = ¼ N0, and T = 5614 years. Plugging these values into the formula, we get:
¼ N0 = N0(1/2)^(3/T)
Solving for t, we get:
t = (3/T) * log(2)
Substituting in T = 5614 years, we get:
t = (3/5614) * log(2) ≈ 1,684 years
Therefore, the cloth is approximately 1,684 years old.
In summary, we can use the concept of radioactive decay and the half-life of Carbon-14 to determine the age of the ancient cloth. By knowing the current amount of Carbon-14 in the cloth, we can calculate the time that has passed since it was first created using a simple formula. In this case, the cloth is approximately 1,684 years old.
Ozone (o3) in the atmosphere can be converted to oxygen gas by reaction with nitric oxide (no). Nitrogen dioxide is also produced in the reaction. What is the enthalpy change when 8. 50l of ozone at a pressure of 1. 00 atm and 25°c reacts with 12. 00 l of nitric oxide at the same initial pressure and temperature? [δh°f(no) = 90. 4 kj/mol; δh°f(no2) = 33. 85 kj/mol; δh°f(o3) = 142. 2 kj/mol]
Answers
The enthalpy change when 8.50 L of ozone at a pressure of 1.00 atm and 25°C reacts with 12.00 L of nitric oxide at the same initial pressure and temperature is -277.5 kJ/mol.
The enthalpy change when 8.50 L of ozone at a pressure of 1.00 atm and 25°C reacts with 12.00 L of nitric oxide at the same initial pressure and temperature can be calculated by the given equation. The balanced equation for the reaction is:2O3(g) + 2NO(g) → 2NO2(g) + 3O2(g)The enthalpy change for the given reaction can be determined using Hess’s law. Hess’s law states that the enthalpy change of a reaction is independent of the route taken, provided that the initial and final conditions are the same.
Since the given reaction can be expressed as a sum of a series of known reactions, Hess’s law can be used to calculate the enthalpy change.Using the given data, the enthalpy change for the reaction can be calculated as follows:δH° = 2 × [ΔH°f(NO2(g))] + 3 × [ΔH°f(O2(g))] - 2 × [ΔH°f(O3(g))] - 2 × [ΔH°f(NO(g))]δH° = 2 × [33.85 kJ/mol] + 3 × [0 kJ/mol] - 2 × [142.2 kJ/mol] - 2 × [90.4 kJ/mol]δH° = - 277.5 kJ/mol
To know more about enthalpy change visit:-
https://brainly.com/question/28039996
#SPJ11
a reactant decomposes with a half-life of 139 s when its initial concentration is 0.331 m. when the initial concentration is 0.720 m, this same reactant decomposes with the same half-life of 139 s.
What is the order of the reaction?
What is the value and unit of the rate constant for this reaction?
Answers
The given reactant follows a first-order reaction. The rate constant value for this reaction is 0.0050 s^-1.
The half-life of a first-order reaction is independent of the initial concentration of the reactant. Hence, the given reactant follows a first-order reaction.
The half-life of a first-order reaction can be related to the rate constant (k) as follows: t1/2 = (ln 2)/k. Using the given half-life value (139 s), we can calculate the rate constant for the reaction.
For the initial concentration of 0.331 M, we have 139 s = (ln 2)/k. Solving for k, we get k = 0.00498 \(s^{-1}\).
For the initial concentration of 0.720 M, we have the same half-life of 139 s. Hence, we can use the rate constant value obtained above to calculate the rate of the reaction. Using the first-order rate law, r = k[A], where [A] is the concentration of the reactant, we get:
r = k[A] = (0.00498 \(s^{-1}\))(0.720 M) = 0.00358 M/s
Therefore, the order of the reaction is first-order, and the rate constant value for this reaction is 0.0050 \(s^{-1}\).
Learn more about order of reactions, below:
https://brainly.com/question/29811492
#SPJ11
Combustion analysis of an unknown compound containing only carbon and hydrogen produced 2.845 g of co2 and 1.744 g of h2o. what is the empirical formula of the compound?
Answers
The empirical formula of the unknown compound is CH₂O.
To determine the empirical formula of the unknown compound, we need to find the mole ratios of carbon and hydrogen in the compound based on the given masses of carbon dioxide (CO₂) and water (H₂O) produced during combustion analysis.
First, we calculate the moles of CO₂ and H₂O produced:
Moles of CO₂ = mass of CO₂ / molar mass of CO₂
= 2.845 g / 44.01 g/mol
= 0.0647 mol
Moles of H₂O = mass of H₂O / molar mass of H₂O
= 1.744 g / 18.015 g/mol
= 0.0968 mol
Next, we determine the mole ratio of carbon to hydrogen by dividing the moles of each element by their respective smallest values:
Carbon: 0.0647 mol / 0.0647 mol = 1
Hydrogen: 0.0968 mol / 0.0647 mol = 1.5
We need to simplify the ratio, so we multiply both values by 2 to obtain whole numbers:
Carbon: 1 × 2 = 2
Hydrogen: 1.5 × 2 = 3
The empirical formula of the unknown compound is CH₂O, indicating that it contains 2 carbon atoms, 3 hydrogen atoms, and 1 oxygen atom.
In summary, the empirical formula of the unknown compound is CH₂O.
For more such questions on empirical formula
https://brainly.com/question/1603500
#SPJ4
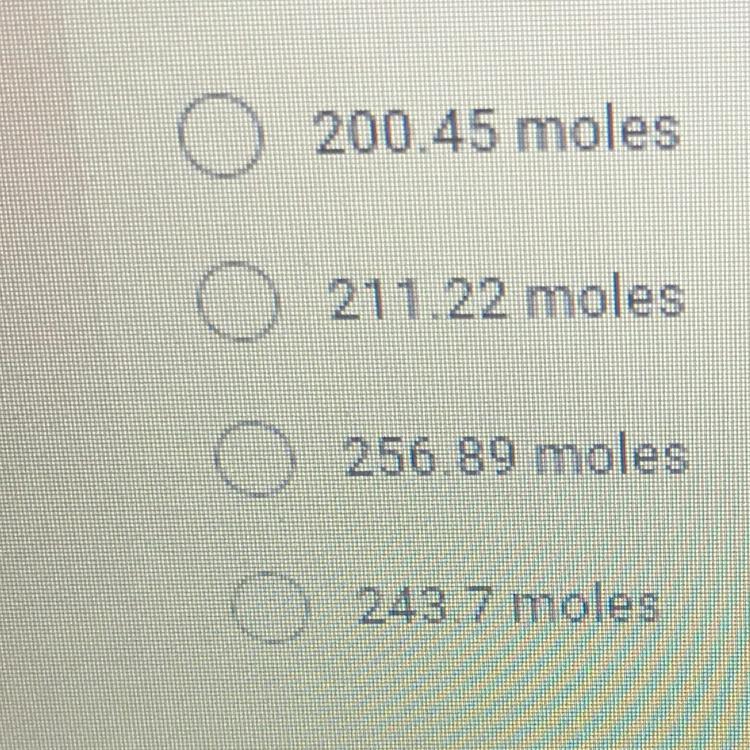
How many moles of gas are in a 30 liter compressed air tank if the
temperature of the tank is 300K and the pressure is 200 atmospheres? *
Answers
Answer:
B
Explanation:
For the reaction
if 5. 0 mol of CO2 are produced, how many moles of O2 were reacted?
a. None of these
b. 3. 3 mol
c. 12. 5 mol
d. 7. 5 mol
e. 6. 2 mol
Answers
If 5.0 mol of the CO₂ are produced, the number of the moles of the O₂ were reacted is 10 mol. The correct option is a. none of these.
The chemical equation is as :
CH₄ + 2O₂ → CO₂ + 2H₂O
The number of the moles of the CO₂ = 5 mol
The number of the moles of the CO₂ = mas / molar mass
The molar mass of the CO₂ = 44 g/mol
The 2 moles of the O₂ produced by the 1 mole of the CO₂
The number of the moles of the O₂ = 2 × 5 mol
The number of the moles of the O₂ = 10 mol.
The number of the moles of the O₂ required to produced 5 mol of the CO₂ is the 10 mol of the O₂. The correct option is a.
To learn more about moles here
https://brainly.com/question/19247935
#SPJ4
A separatory funnel contains ethyl acetate and an aqueous solution of some kind. What comprises the bottom layer?.
Answers
A separatory funnel contains ethyl acetate and some sort of aqueous solution. The bottom layer is made up of methylene chloride.
What is methylene chloride ?Methylene chloride, a clear, colorless, nonflammable, volatile liquid chlorinated hydrocarbon with a sweet, agreeable odor, releases highly poisonous phosgene vapours when heated to the point of disintegration.Methylene chloride is primarily used as a solvent in paint removers, but it is also used in aerosol formulations, pharmaceutical production, surface degreasing, electronic manufacturing, and ethane foam blowing.Dichloromethane can be found naturally in the ocean, macroalgae, marshes, and volcanoes. Industrial emissions, on the other hand, are the primary source of dichloromethane in the environment. The end products of these reactions are chloromethane, dichloromethane, chloroform, carbon tetrachloride, and hydrogen chloride. These chemicals are separated using distillation.To learn more about methylene chloride, refer to:
brainly.com/question/13189086
#SPJ4
transfer function of a passive filter with the rejection range of (2/t) hz is given as h(s)-(2s 128)/(s as b), for this filter:
Answers
To analyze the given transfer function, h(s) = (2s + 128) / (s^2 + as + b), we need to determine the values of a and b, which will define the behavior of the filter.
The transfer function represents a second-order passive filter. To find the values of a and b, we can compare the given transfer function with the general form of a second-order transfer function:
h(s) = ωn^2 / (s^2 + 2ζωn s + ωn^2),
where ωn is the natural frequency and ζ is the damping ratio.
By comparing the given transfer function with the general form, we can equate the coefficients:
s^2 + as + b = s^2 + 2ζωn s + ωn^2.
From this equation, we can determine the values of a and b as follows:
1. The coefficient of s in the given transfer function is 2, while the general form has 2ζωn. Therefore, we have:
2 = 2ζωn.
2. The constant term in the given transfer function is 128, while the general form has ωn^2. Therefore, we have:
b = ωn^2.
Now, we have two equations:
2 = 2ζωn,
b = ωn^2.
Since we don't have specific values for ωn and ζ, we cannot determine the exact values of a and b. We need additional information or specifications to calculate those values.
The given transfer function provides the numerator and denominator coefficients but does not provide enough information to determine the specific values of a and b.
To know more about function refer here
https://brainly.com/question/30721594#
#SPJ11
Which of the following best explains many physical properties of a material?
Answers
The arrangement of its atoms or molecules best explains many physical properties of a material.
What is Metal?
A metal is a type of material that is typically hard, shiny, malleable, ductile, and has good electrical and thermal conductivity. Metals are characterized by their ability to form cations, which are positively charged ions, by losing electrons. They typically have metallic bonds, which are formed when the outermost electrons of metal atoms are delocalized and shared among a lattice of metal ions. This results in metals having a unique set of physical and chemical properties, such as high melting and boiling points, high density, and good strength-to-weight ratios, making them useful for a wide range of applications, including construction, transportation, electronics, and manufacturing.
Many physical properties of a material can be explained by its atomic and molecular structure. The arrangement of atoms and molecules in a material determines its physical properties such as density, melting and boiling points, thermal conductivity, electrical conductivity, and more. For example, a material with a tightly packed atomic arrangement will be denser than a material with a more open arrangement. Similarly, the strength of the bonds between atoms and molecules will determine how much energy is required to melt or boil the material.
Learn more about Metal from given link
https://brainly.com/question/4701542
#SPJ1
Which of the following compounds do not contain an sp3 hybridized oxygen atom? _ A A) ketones B) alcohols C) ethers D) esters E) water
Answers
Which of the following compounds do not contain an sp3 hybridized oxygen atom is option E) water. The oxygen atom in an sp3 hybridized state has a tetrahedral arrangement of its bonds, which is achieved through the hybridization of one s orbital and three p orbitals.
This creates four identical sp3 hybrid orbitals that point to the vertices of a tetrahedron. Each of the listed chemical substances has an oxygen atom bonded to other atoms or functional groups.
The following is a brief explanation of each of them: Ketones contain a carbonyl group, which consists of a carbon atom with a double bond to an oxygen atom, and two additional carbon-containing groups. As a result, the oxygen atom in a ketone is sp2 hybridized, with one lone pair in an unhybridized p orbital.Alcohols have an -OH functional group, which consists of an oxygen atom covalently bonded to a hydrogen atom and a carbon-containing group. The oxygen atom in an alcohol is also sp3 hybridized.
Ethers have two carbon-containing groups connected to an oxygen atom. The oxygen atom in an ether is sp3 hybridized.Esters have a carbonyl group and an alkoxy group (-OR). The oxygen atom in an ester's carbonyl group is sp2 hybridized, while the oxygen atom in the -OR group is sp3 hybridized. Water, on the other hand, is composed of two hydrogen atoms and an oxygen atom, with no other atoms connected to the oxygen atom. In water, the oxygen atom is also sp3 hybridized, with two lone pairs and two single bonds to hydrogen atoms. Therefore, Option E is correct.
To know more about Alcohols visit-
https://brainly.com/question/29268872
#SPJ11
Calculate the mass of CuO which can react with 39,2 grams of orthophosphate acid.Please Help!!3CuO+ 2H3PO4 = Cu3(PO4)2 + 3H20
Answers
Answer
47.73 g CuO
Explanation
Given:
Chemical equation: 3CuO+ 2H3PO4 = Cu3(PO4)2 + 3H20
mass of orthophosphate acid (Cu3(PO4)2) = 39.2 g
Required: The mass of CuO
Solution:
\(\begin{gathered} 39.2g\text{ H}_3PO_4\text{ x }\frac{1\text{ mol H}_3PO_4}{97,994g\text{ H}_3PO_4}\text{ x }\frac{3\text{ moles CuO}}{2\text{ moles H}_3PO_4}\text{ x }\frac{79,545g\text{ CuO}}{1mole\text{ CuO}} \\ \\ =\text{ 47.73 g CuO} \end{gathered}\)Second method:
Step 1: Find the moles of H3PO4
n = m/M where m is the mass and M is the molar mass of H3PO4
n = 39.2g/97.994g.mol^-1
n = 0.400 mol
Step 2: Use the stoichiometry to find the moles of CuO
The molar ratio between CuO and H3PO4 is 3:2
Therefore the moles of CuO = 0.400 mol x (3/2) = 0.600 mol
Step 3: Find the mass of CuO, now that we have moles
m = n x M m is the mass, n is the moles and M is the molar mass
m = 0.600 mol x 79,545 g/mol
m = 47.73 g
The observed variance, 2obs, for a peak is the sum of the variances from all contributing factors. The following contributes to 2obs
- 2 column
- 2 detector
- 2 injection
- 2 tubing
Answers
The observed variance (2obs) is the sum of the variances contributed by these four factors, i.e., 2obs = 2column + 2detector + 2injection + 2tubing.
What is a variance?It seems that the observed variance (2obs) for a peak is the sum of the variances from all contributing factors. According to the information provided, the following factors contribute to 2obs:
Column: This refers to the chromatography column used in the separation process. The properties of the column, such as its length, diameter, and stationary phase, can affect the variance of the peak.
Detector: The detector used in the analysis can also contribute to the observed variance. The sensitivity, noise level, and other characteristics of the detector can influence the variance.
Injection: The way the sample is introduced into the system can affect the peak variance. The injection technique, injection volume, and other factors can contribute to the overall variance.
Tubing: The tubing used in the system can also contribute to the peak variance. The diameter, length, and material of the tubing can affect the variance.
Therefore, the observed variance (2obs) is the sum of the variances contributed by these four factors, i.e., 2obs = 2column + 2detector + 2injection + 2tubing.
Learn more about variance
brainly.com/question/31432390
#SPJ11
As a result of this process, the proportions of oxygen and carbon dioxide in
air breathed in and air breathed out change.
Which one of the statements is true? Tick the correct box. [1]
- Air breathed out has less carbon dioxide and more oxygen than air breathed in.
- Air breathed out has less carbon dioxide and less oxygen than air breathed in.
- Air breathed out has more carbon dioxide and less oxygen than air breathed in.
- Air breathed out has more carbon dioxide and more oxygen than air breathed in.
Answers
Answer:
the third one
Explanation:
When you breathe in, you inhale oxygen and exhale carbon dioxide
One step in the production of copper is to heat copper (I) sulfide, Cu2S, with oxygen. This
produces copper (I) oxide and sulfur dioxide gas according to the following reaction:
2Cu2S(s)
+ 3O2(g)
→ 2Cu2O(s)
+ 2SO2(g)
What is the greatest mass of SO2 that can be produced from 15.0 mol Cu2S?
Answers
Answer:
9.61 x 10^2 g SO2
Explanation:
Ur welcome
Lab: Measuring pH - Assignment: Lab Report ODL Chemistry
PLEASE HELP
100 points!!!
Answers
Here are the typical sections that you should include in your report:
Title Page - This page should contain the title of your experiment, your name, your instructor's name, and the date the experiment was conducted.
Introduction - In this section, you should provide some background information on pH and why it is important to measure it. You should also state the objectives of the experiment and describe the methodology you used.
Materials and Methods - This section should include a list of all the materials you used in the experiment, as well as a step-by-step description of the procedure you followed to measure pH. Be sure to include any safety precautions you took during the experiment.
Results - In this section, you should present the data you collected during the experiment. You should include tables, graphs, or figures to illustrate your results. You should also provide a written explanation of your findings.
Discussion - In this section, you should interpret your results and explain what they mean in terms of the objectives of the experiment. You should also discuss any sources of error that may have affected your results, and suggest ways to improve the experiment in the future.
Conclusion - In this section, you should summarize your findings and state whether or not your objectives were achieved.
References - This section should include a list of any sources you consulted during the experiment, such as textbooks, journal articles, or websites.
Appendices - This section should include any additional information that is relevant to the experiment but not included in the main body of the report, such as raw data, calculations, or photographs.
When writing your lab report, be sure to follow the formatting and citation guidelines provided by your instructor or department. You may also want to consult a scientific writing guide or other resources to help you write a clear and concise report.
\(\begin{align}\huge\colorbox{black}{\textcolor{yellow}{I hope this helps !}}\end{align}\)
\(\begin{align}\colorbox{purple}{\textcolor{lime}{Please mark as brillinest !}}\end{align}\)
\(\textcolor{cyan}{\small\textit{If you have any further questions, feel free to ask!}}\)
Energy has different _____ , such as electrical, chemical, and thermal.
Answers
Answer:
forms/types
Explanation:
Select the correct empirical formula for each molecular formula given.
Molecular formula: C6H12O6
Empirical formula:
C6H12O6
C2H4O2
CH2O
CHO
Answers
Answer: CH2O
Explanation:
this formula is glucose, as you said the molecular formula is C6H12O6 because an actual molecule of glucose contains that many atoms of each element
an empirical formula is the simplest whole-number ratio of a formula (ex: 1-2-1)
What is the pressure exerted by 32g of o2 in a 22.0L container at 30.0 degrees Celsius
Answers
Answer:
114 kPa
Explanation:
Using the ideal Gas Law, derive an expression relating the concentration of a gas μg
−3
(X) with the concentration expressed in ppm (Y). (10 pts) Hint: - Ideal gas law PV=nRt −R=0.0821 atm mol
−1
−MW=X= molecular weight
Answers
The expression relating the concentration of a gas in \(\mu g/m^-^3 (X)\) with the concentration expressed in ppm (Y) is \(Y = (X/MW) * 10^6 / V\)
To derive an expression relating the concentration of a gas in \(\mu g/m^3 (X)\) with the concentration expressed in ppm (Y), we can use the ideal gas law. The ideal gas law states that \(PV = nRT\), where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature. Rearranging the equation, we have \(n = PV/RT\)
To convert the concentration from \(\mu g/m^-^3 (X)\) to moles (n), we divide X by the molecular weight (MW) of the gas. Thus, \(n = X/MW\)
Combining the two equations, we have \(X/MW = PV/RT\)
Since the concentration expressed in ppm (Y) is the same as the number of moles per million parts of air, we can write \(Y = n * 10^6 / V\)
Substituting \(n = X/MW\), we get \(Y = (X/MW) * 10^6 / V\)
Therefore, the expression relating the concentration of a gas in \(\mu g/m^3 (X)\) with the concentration expressed in ppm (Y) is:
\(Y = (X/MW) * 10^6 / V\)
Learn more about ideal gas law here:
https://brainly.com/question/30458409
#SPJ11
Complete question is:
Using the ideal Gas Law, derive an expression relating the concentration of a gas \(\mu_g^-^3 (X)\) with the concentration expressed in ppm (Y). (10 pts) Hint:- Ideal gas law \(PV= nRt -R = 0.0821 atm mol^-^1\) −MW=X= molecular weight
Kilograms represented by the mass defect for oxygen-16: 2.20 × 10 -28 kg what is the nuclear binding energy for oxygen-16? 3.0 × 108 j 6.60 × 10-20 j 1.98 × 10 -11 j 3.69 x 10-24 j
Answers
From the calculation, the binding energy of the oxygen atom is 1.98 * 10^11 J
What is the binding energy?The term binding energy has to do with the energy that must be supplied to the nucleus of an atom for the nucleus of the atoms to be bonded together.
We must note that;
E = mc^2
E = 2.20 × 10 -28 kg * ( 3 * 10^8m/s)^2 = 1.98 * 10^11 J
Learn more about binding energy:https://brainly.com/question/10095561
#SPJ4
Answer:
1.98 × 10 -11 J
Explanation:
edg
Why are earthworms called as “friends of farmers”? Answer in no less than 4 sentences
Answers
Answer:
Organic matter composted with earthworms, often known as vermicompost, has been demonstrated in studies to have disease-suppressing qualities. The earthworms loosen the soil and enrich it with humus. As a result, they are known as farmer's pals. They help the crops grow healthier and over the time have earned farmers trust.
Explanation:
Hope this helps in some way!
what mining biodivirsity
Answers
Answer:
Explanation:
Mining affects biodiversity at multiple spatial scales (site, landscape, regional and global) through direct (i.e. mineral extraction) and indirect processes (via industries supporting mining operations, and external stakeholders who gain access to biodiversity-rich areas as the result of mining).
Victor put a match on a balance and found that its mass was 6.0 grams. Then he lit the match and put it out after a few seconds.
He put the partially burned match on the balance again. This time, its mass was 4.5 grams.
Which of the following best explains what happened as the match burned?
A.
Some of the matter changed from solid to gas and went into the air.
B.
The matter moved to a different part of the match.
C.
Some of the mass was destroyed by the fire.
D.
The mass of the match combined with the mass of the fire.
Answers
Place the cursor at the start of the text, then press CTRL+SHIFT+DOWN ARROW. Press CTRL+SHIFT+UP ARROW after advancing the cursor to the end of the paragraph. The Correct option is B.
How do you move the first sentence to the end of the paragraph in Word?The Ctrl+A keyboard shortcut in Word allows you to select all text in a document, and you can also use the mouse or keyboard to pick out individual words or objects from tables. Text or objects that are located in various locations can also be chosen. You can choose, for instance, a sentence from one page and a paragraph from another.The entire textIn the document, click anywhere.You can pick every word in the document by pressing Ctrl+A on your computer.The particular wording you wantAdditionally, you have the option of choosing a specific word, line of text, or one or more paragraphs.Your cursor should be placed in front of the first letter of the word, sentence, or paragraph you want to pick.To pick a text block, click and hold while dragging the cursor over it.Other options for selecting textQuickly click and drag the mouse over a word to choose it.By positioning your cursor at the beginning of a line of text and pressing Shift + down arrow, you can pick that line of text.Put your cursor at the beginning of the paragraph, then hit the down arrow while holding down the Ctrl and Shift keys to select the paragraph.To Learn more About end of the paragraph refer to:
https://brainly.com/question/25717629
#SPJ1