URGENT !! I'LL GIVE BRAINLIEST IF YOU WANT! 100 POINTS
Which statement is NOT TRUE about molecules and compounds?
A. Compounds are made up of the same type of atom.
B. Molecules are made up of two or more chemically bonded atoms.
C. All molecules and compounds are made of atoms.
D. All compounds are molecules.
Answers
The statement that is not true about molecules and compounds is compounds are made up of the same type of atom. The correct option is A.
In chemistry, molecules and compounds are fundamental ideas. Two or more atoms that are chemically linked together make up a molecule.
A specific class of molecule known as a compound is made up of various atom types that are chemically combined in predetermined ratios.
As the fundamental units of matter, atoms make up all molecules and compounds. However, it is untrue to say that all compounds contain the same kind of atom.
While molecules can be made up of atoms from the same element or from different elements, compounds are created by the mixing of various types of atoms.
Thus, the correct option is A.
For more details regarding compounds visit:
https://brainly.com/question/34151797
#SPJ3
Related Questions
how many electrons are there in 2 g of H2
Answers
Answer:
6.02 x 10²³ electrons
Explanation:
Given parameters:
Mass of H₂ = 2g
Unknown:
Number of electrons = ?
Solution:
To find the number of electrons, we must determine the number of moles of H₂ first.
Number of moles = \(\frac{mass}{molar mass}\)
Molar mass of H₂ = 2(1) = 2g/mol
Number of moles = \(\frac{2}{2}\) = 1mol
1 mole of a substance contains 6.02 x 10²³ particles
the particles can be protons, neutrons, electrons
So,
2g of H₂ will contain 6.02 x 10²³ electrons.
Students are copying their homework assignment from the whiteboard in their dimly lit classroom Some students are wearing eyeglasses, some are not wearing anything over their eyes, and
one student is wearing dark sunglasses. Which material allows the least light to pass through it so that the smallest amount of light reaches the student's eyes?
Eyeglasses
Sunglasses
Air
Answers
Answer:
sunglasses
Explanation:
what change will be caused by addition of a small amount of hclo4 to a buffer solution containing nitrous acid, hno2, and potassium nitrite, kno2? group of answer choices
Answers
A buffer solution is defined as a solution that resists a change in pH when a small amount of acid or base is added to it. the buffer capacity of the solution will prevent the pH from changing too much.
The buffer solution containing nitrous acid, HNO2, and potassium nitrite, KNO2, will experience the following changes when a small amount of HClO4 is added to it: The HClO4 added to the buffer solution will react with the potassium nitrite, KNO2, to form the salt, KClO4.T
he HNO2 will be converted to nitric acid, HNO3, by the HClO4.The HNO3 formed in the previous step will react with the potassium nitrite, KNO2, to form nitric oxide, NO, and potassium nitrate, KNO3.The net effect of adding HClO4 to the buffer solution containing nitrous acid, HNO2, and potassium nitrite, KNO2, will be to shift the buffer solution to a more acidic pH range.
However, the buffer capacity of the solution will prevent the pH from changing too much.
To know more about buffer solution visit
https://brainly.com/question/31428923
#SPJ11
Help needed ASAP, I will mark your answer as brainliest.

Answers
Calculate the Current required the Produce 20dm³ of chlorine gas at STP by electrolysis for one hour?
Answers
The current involved is 44.7 A
What is the current required?In an electrolytic reaction, we have the situation in which one specie is oxidized and the other specie is reduced in the electrochemical reaction. We have the reaction that occurs as;
2Cl^-(aq) ------> Cl2(g) + 2e
Now;
If 2 * 96500 C liberates 22.4 dm^3 of Cl2
x C liberates 20dm³ of chlorine gas at STP
x = 2 * 96500 C * 20dm³/24 dm³
x = 160833.33 C
Now
Q = It
Q = quantity of electricity
I = current
t = time = 1 hour or 3600 s
I = Q/t = 160833.33 C/ 3600 s
I = 44.7 A
Learn more about electrolysis:https://brainly.com/question/12054569
#SPJ1
Which of the following would form a precipitate?
A. Li2CO3
B. AgBr
C. Cr(NO3)2
D. NaOH
Answers
Therefore, the compound that would form a precipitate is option D, NaOH.
What is precipitate?A precipitate is a solid that forms from a chemical reaction between two or more liquids or solutions. This solid typically forms when two solutions are mixed together and a chemical reaction occurs that causes one of the products to become insoluble in the mixture, resulting in a solid that falls out of the solution and settles at the bottom. Precipitates can vary in size, shape, and color depending on the specific chemical reaction and the properties of the substances involved.
Here,
To determine which of the given compounds would form a precipitate, we need to know the solubility rules for common ionic compounds. Here are the rules for the given ions:
Li2CO3: carbonates are generally insoluble, but Li2CO3 is an exception and is soluble in water
AgBr: silver halides (AgCl, AgBr, AgI) are generally insoluble, so AgBr would form a precipitate
Cr(NO3)2: nitrates (NO3-) are generally soluble, so Cr(NO3)2 would dissolve in water and not form a precipitate
NaOH: hydroxides (OH-) of Group 1 and most Group 2 metals are soluble, but NaOH is an exception and is only slightly soluble, so it would form a precipitate
To know more about precipitate,
https://brainly.com/question/30904755
#SPJ1
Using the notation just described, represent a cell based on the following reaction:
ClO3−(aq)+3Cu(s)+6H+(aq)→Cl−(aq)+3Cu2+(aq)+3H2O(l)
Pt is used as an inert electrode in contact with the ClO3− and Cl− .
Drag the appropriate labels to their respective targets. For neutral species, leave the pink target blank.

Answers
The cell notation of the redox reaction between Cu and ClO₃⁻ is:
Cu/Cu²⁺//ClO₃⁻//Cl⁻/PtWhat is a voltaic cell?A voltaic cell or electrochemical cell is a device which produces electricity from chemical reactions occuring within the cell.
The reactions occuring in a voltaic cell are redox reactions.
Oxidation occurs at the anode while reduction occurs at the cathode.
In the given reaction between Cu and ClO₃⁻ as shown below:
ClO₃⁻ (aq) + 3 Cu (s) + 6H⁺ (aq) → Cl⁻ (aq) + 3 Cu²⁺ (aq) + 3 H₂O (l)Cu is oxidized as follows: 3 Cu (s) → 3 Cu²⁺
ClO₃⁻ is reduced as follows: ClO₃⁻ (aq) → Cl⁻ (aq)
The voltaic cell notation is as follows where Pt is used as an inert electrode in contact with the ClO₃⁻ and Cl⁻:
Cu/Cu²⁺//ClO₃⁻//Cl⁻/PtTherefore, the cell notation of the redox reaction between Cu and ClO₃⁻ shows that Cu is oxidized while ClO₃⁻ is reduced.
Leran more about voltaic cells at: https://brainly.com/question/3930479
g a substance that heats up relatively quickly has a question 8 options: high specific heat. low specific heat. low conductivity. high conductivity.
Answers
The substance heats up relatively quickly means less energy needed for the rise in temperature. Therefore, the substance is having low specific heat.
Of all liquids, water has the highest specific heat capacity. The amount of heat that one gram of a substance needs either absorb or lose in order to change its temperature by one degree Celsius is known as specific heat. A compound with "low specific heat" will raise its temperature considerably more quickly than a compound with "high specific heat." J/kg C or J/g C are the units for specific heat. While substances with (high or low) particular heats will be more challenging to heat up, substances with (high or low) specific heats will heat up readily.
To learn more about specific heat capacity visit:https://brainly.com/question/28302909
#SPJ4
Please help due today!

Answers
Answer:
It's the first answer choice for sulfur and the second answer choice for magnesium I believe
Questions: Answer on the following slide.
1. What is needed from adults when conducting experiments at home?
2. Why are chemicals so dangerous?
3. What should always be followed when conducting experiments?
4. What is science?
Answers
what is the type of weak bond between the hydrogen of one molecule and the nitrogen of another molecule, where the two don't actively share an electron? group of answer choices hydrogen bond ionic bond disulfide bond hydrophobic bond covalent bond
Answers
The weak bond between the hydrogen atom of one molecule and the nitrogen atom of another molecule, where the two don't actively share an electron, is called hydrogen bond. Option A is correct.
A hydrogen bond is a type of intermolecular attraction that occurs when a hydrogen atom, already covalently bonded to one electronegative atom, interacts with another electronegative atom.
In this case, the hydrogen is bonded to a highly electronegative nitrogen atom, creating a partially positive charge on the hydrogen and a partially negative charge on the nitrogen, allowing for electrostatic attraction between the two molecules.
Hydrogen bonds are relatively weak compared to covalent bonds, but they play a critical role in many biological and chemical processes. For example, hydrogen bonds help hold together the two strands of DNA, which is critical for the proper functioning of genetic information.
Hence, A. is the correct option.
To know more about hydrogen bond here
https://brainly.com/question/10904296
#SPJ4
--The given question is incomplete, the complete question is
"What is the type of weak bond between the hydrogen of one molecule and the nitrogen of another molecule, where the two don't actively share an electron? group of answer choices A) hydrogen bond B) ionic bond C) disulfide bond D) hydrophobic bond E) covalent bond."--
Plz answer these three questions I’m really confused about it

Answers
Which statement about atoms during a chemical change is true?
Answers
Answer:
In a chemical reaction, only atoms are present in the reactant and can end up with molecules. No new atoms are created and no more atoms are destroyed. In a chemical reaction, reactants contact with each other and the bond between atoms in the reactant is broken and atoms rearrange and form new bonds to make the product.
Explanation:
15. Discuss the various factors which affect the rate of evaporation. Latent heat
evaporation of two liquids A and B is 100 J/kg and 150 J/kg respectively. Which
can produce more cooling effect and why? (5)
Answers
Answer:
The correct answer is liquid B.
Explanation:
Latent heat of vaporization also known as the latent heat of evaporation. This latent heat transforms the particles of liquid into a gas without affecting its temperature. For example, the latent heat of evaporation for water is 40.8 kJ per mole, that is, 40.8 kJ per mole of heat is needed to transform water into vapor at 373 K.
It is known that latent heat of evaporation of a liquid is directly proportional to the cooling effect it generates, that is, more the latent heat of evaporation more will be its cooling effect. Thus, it is clear that liquid B will show the more cooling effect as the latent heat of evaporation of liquid B is more in comparison to liquid A. Thus, more heat will be captivated by liquid B and will generate more cooling effect in comparison to liquid A.
a log is added to a camp fire and is bured. this is an example of a change.
Answers
Answer:
Chemical Change
Explanation:
A log is added to a camp fire and is burned. This is an example of a chemical change.
A gas has a solubility of 0.086 g/l at a pressure of 3.5 atm. At what pressure would the solubility be at 2.3 g/L?
Answers
The pressure at which the the gas has a solubility of 2.3 g/L is 93.95 atm
How to find the pressure?To find the pressure at which the solubility would be 2.3 g/L, we need to use equation for Henry's law, which is written as:
S₁/P₁ = S₂/P₂
Where the variables in that equation are:
S₁ = Initial solubilityP₁ = Initial pressureS₂ = Final solubilityP₂ = Final pressureWe know the values of:
S₁ = 0.086 g/L
P₁ = 3.5 atm
S₂ = 2.3 g/L
Solving the equation for P₂:
S₁/P₁ = S₂/P₂
P₂ = (S₂ * P₁) / S₁
Now evaluate it to get:
P₂ = (2.3 g/L * 3.5 atm) / 0.086 g/L
P₂ = 93.95 atm
Learn more about solubility at:
https://brainly.com/question/23946616
#SPJ1
a flat metal surface in an electric cell is its _________ terminal
Answers
Answer:
negative
Explanation:
what is the mass of one mole of magnesium chloride
Answers
The mass of one mole of magnesium chloride is 95.21 g/mol.
The mass of one mole of magnesium chloride (MgCl2) can be calculated by adding the atomic masses of one mole of magnesium and two moles of chlorine.
Atomic mass of magnesium = 24.31 g/mol . Atomic mass of chlorine = 35.45 g/mol (because chlorine has two isotopes, the average atomic mass is taken) . Thus, the mass of one mole of magnesium chloride (MgCl2) = 24.31 g/mol + 2(35.45 g/mol) = 24.31 g/mol + 70.90 g/mol = 95.21 g/mol . Therefore, the mass of one mole of magnesium chloride is 95.21 g/mol.
Atomic mass is the amount of matter that makes up an element's atom. The total mass of the protons, neutrons, and electrons that make up an atom is a little bit less than the observable atomic mass.
The average mass of an element's atoms , expressed in ( amu, commonly known as daltons , D) is the element's atomic mass. The mass of each isotope is multiplied by its abundance to get the atomic mass , which is a weighted average of all the isotopes of that element.
To know more about Atomic mass :
https://brainly.com/question/26609073
#SPJ11
Create a visual model of an ionic substance (salt) dissolving in water and a covalent substance (sugar) dissolving in water.
Answers
Answer:
see image
Explanation:
let me know if you have any questions

When we react an acid with a base a neutralisation reaction occurs.
What pH do you end up with?
Answers
Explanation:
Good question!
When you react an acid with a base,the pH of the product is determined by the concentrations of the reactants (the acid and the base)
If both reactants (acid and base) are strong,the pH of the product is 7
If the acid is stronger than the base,the pH of the product will be less than 7
If the base is stronger,the pH of the product will be greater than 7
I hope this helps
calculate the isoionic and isoelectric ph of 0.028320.02832 m valine. enter your answers to the hundredths place.
Answers
Answer:
isoelectronic pH = 6.003
Explanation:
isoelectronic pH = (pKa1+pKa2)/2
How does the valency of an element show its reactiveness in a chemical reaction
Answers
Answer:
Within each group of metals, reactivity increases as you go down the group. The valence electrons are less tightly bound and easier to remove, because they are farther away from the nucleus of the atom. A nonmetal tends to attract additional valence electrons to attain a full valence shell.
Hope it's helpful to you
Please help it’s the end of the semester test and it’s also science by the way but there is no science subjects on here for some reason so I just picked chemistry.

Answers
Answer:
Battery
Mobile phone
Explanation:
The items that are matter are the battery and the mobile phone.
Matter is anything that has weight and occupies space.
There are three known states of matter which are solids, liquids and gases. A battery and mobile phone are solid states of matter. Electricity, sound, light and heat are all forms of energy. They are not substantial and they characterize matter. They lack the properties that make a matter.A gas is contained in a cylinder with a volume of 4.3 L at a temperature of 30.3oC and a pressure of 766.9 torr. The gas is then compressed to a volume of 0.38 L, and the temperature is raised to 839.0oC. What is the new pressure of the gas? Express your answer in atmospheres (atm)
Answers
The ideal gas law can be used to determine the gas's new pressure. According to the ideal gas law, pressure times volume equals the amount of a gas in moles times the global gas constant times the temperature.
Pressure = (number of moles of gas times the universal gas constant times the temperature) divided by volume is the new equation we may use to calculate pressure.
Since there are an equal amount of moles of gas in this situation, we can use the following formula to determine the new gas pressure: Pressure is equal to (4.3 L times 839.0oC) divided by (766.9 torr times 0.38 L). We obtain a new pressure of 118.6 atm by simplifying.
Learn more about pressure at:
https://brainly.com/question/12971272
#SPJ1
PLEASE I NEED HELP I WILL GIVE YOU BRAINLIST

Answers
Answer:
First Q :B
Second Q:C
SURELY SOMEONE HELP it’s urgent plllss I’ll brainlist u/5 star!!! answer the ones u know. :)
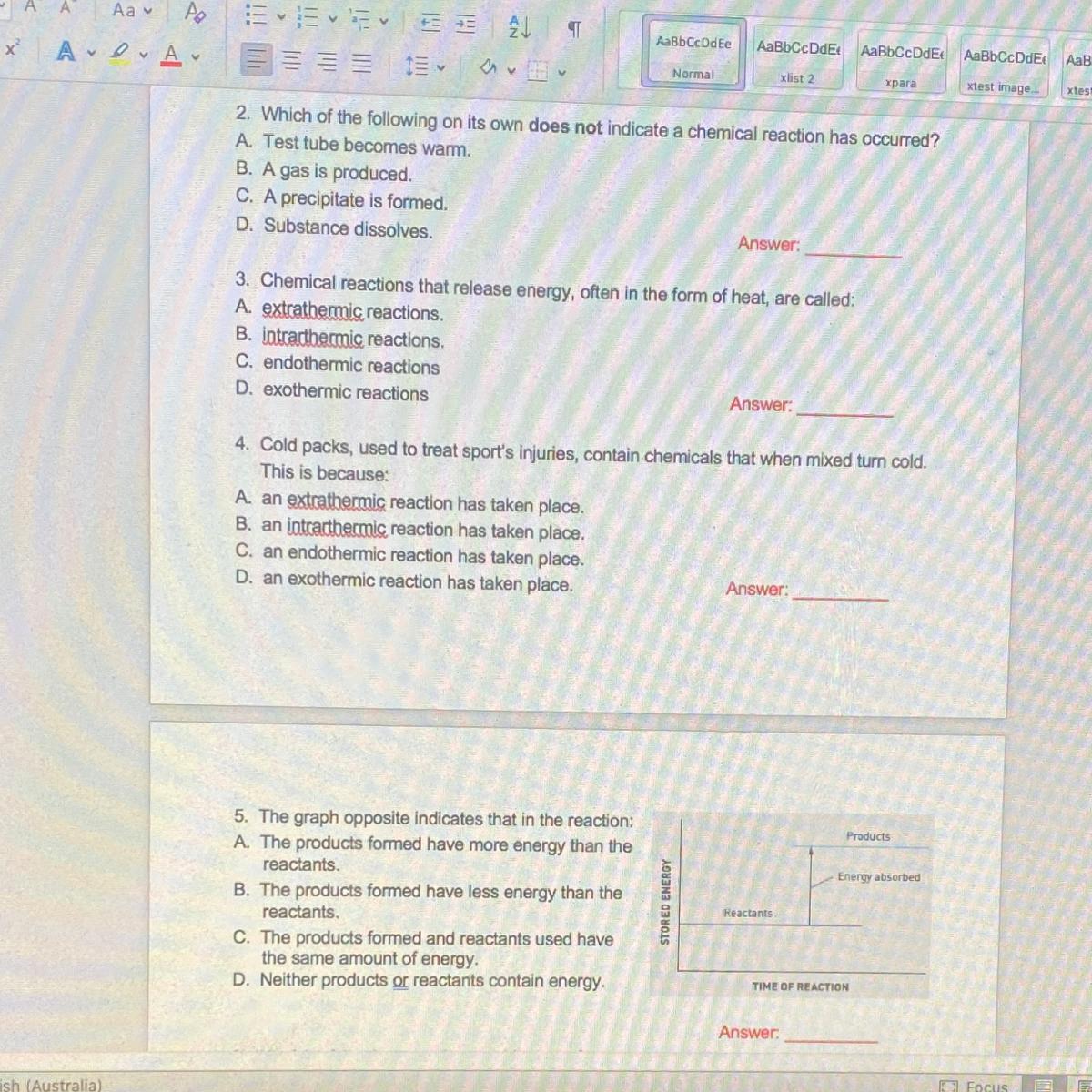
Answers
Answer:
3 exothermic reaction. only that much
Hi,
These are the answers.
• Question 2. C , Substance dissolves
• Question 3. D , Exothermic reaction
• Question 4. C , Endothermic reaction takes place
• Question 5. A , the products formed has more energy than reactants
Hope it helps you... pls mark brainliest if it helped you
an isotope of gallium, 67ga, has an atomic number of 31 and a half-life of 78 hours. consider a small mass of 3.2 grams for 67ga which is initially pure. 1)initially, what is the half-life of the gallium? t1/2o
Answers
The half-life is a constant property of an isotope and does not change based on the mass or purity of the sample.
The initial half-life of 67Ga is given as 78 hours. This means that after 78 hours, the mass of 67Ga will be reduced to half of its initial value. Gallium-67 (67Ga) is an isotope of gallium with an atomic number of 31 and a half-life of 78 hours. When considering a small mass of 3.2 grams of initially pure 67Ga, the initial half-life (t1/2o) remains the same as the half-life of this particular isotope, which is 78 hours. The half-life is a constant property of an isotope and does not change based on the mass or purity of the sample. When considering a small mass of 3.2 grams of initially pure 67Ga, the initial half-life (t1/2o) remains the same as the half-life of this particular isotope, which is 78 hours.
To know more about isotope visit:
https://brainly.com/question/28039996
#SPJ11
3. Which graph best matches a person walking away slowing and returning quickly!
А
B.
D
distance
time
time
time
time

Answers
Answer:B
Explanation:
Balance the equation !!!

Answers
Answer:
https://www.webqc.org/balance.php
Explanation:
this is the website i used to balance all my chem equations
i promise its not a scam its a legit website lol it works rly well
Suppose 125 mL of a 0.020 M Pb(NO3)2 solution is mixed with 75 mL of a 0.020 M NaClNaCl solution. Will a precipitate form? The KspKsp for PbCl2 is 1.6×10^−5
Answers
No precipitate will form, as the system is not yet at equilibrium and there is still room for more PbCl2 to dissolve.
To determine if a precipitate will form when mixing the solution, we need to calculate the ion product, Q, and compare it to the solubility product, Ksp.
First, let's write out the balanced equation for the reaction between Pb(NO3)2 and NaCl:
Pb(NO3)2 + 2NaCl → PbCl2 + 2NaNO3
Since we're dealing with a 0.020 M solution of each salt, we can use the concentration and volume to calculate the moles of each ion present:
moles of Pb2+ = (0.020 M) x (0.125 L) = 0.0025 mol
moles of Cl- = 2 x (0.020 M) x (0.075 L) = 0.003 mol
Now we can calculate the ion product, Q:
Q = [Pb2+][Cl-]^2 = (0.0025 M)(0.003 M)^2 = 2.25 x 10^-8
Comparing Q to the Ksp value of 1.6 x 10^-5, we see that Q is less than Ksp, thus no precipitate will be seen.
Learn more about solution : https://brainly.com/question/25326161
#SPJ11