two moles of carbon dioxide gas at 35°c are heated to 250°c in a container while the volume is kept constant. the density of the gas in the container will
a. Increase
b. Decrease
c. Remain the same
d. Reach a value of zero
e. There is not enough information given to correctly answer this question
Answers
Carbon dioxide (chemical formula CO2) is a chemical compound made up of molecules that each have one carbon atom covalently double-bonded to two oxygen atoms. It is found in the gaseous state at room temperature, and as the source of available carbon in the carbon cycle, atmospheric CO2 is the primary carbon source for life on Earth.
Carbon dioxide is a chemical element that can be found in the atmosphere. At room temperature, it is a gas. It has one carbon atom and two oxygen atoms. When people and animals exhale, carbon dioxide is released. It is a greenhouse gas that is found in low concentrations in the Earth’s atmosphere. Dry ice is what it is when it is firm.
The density of the gas in the container when two moles of carbon dioxide gas at 35°C are heated to 250°C while the volume is kept constant will:
b. Decrease
Here's a step-by-step explanation:
1. At constant volume, the pressure of the gas is directly proportional to its temperature (Gay-Lussac's Law).
2. When the temperature increases from 35°C to 250°C, the pressure of the gas will also increase.
3. Density is given by the equation ρ = m/V, where m is mass and V is volume.
4. Since the volume is constant and the number of moles remains the same, the mass of the gas does not change.
5. However, as the pressure increases, the gas particles move further apart, causing a decrease in density.
To know more about the (Gay-Lussac's Law) https://brainly.com/question/2683502
#SPJ11
Related Questions
What is the answer?

Answers
Answer:
Option A
Explanation:
The atomic number is the number of protons in the nucleus of an atom.
If the free energy change delta G for a reaction is -46.11 kJ/mol, the reaction is:A) at equilibrium.B) endergonic.C) endothermic.D) exergonic.E) exothermic.
Answers
The reaction is exergonic. that is the free energy change delta G for a reaction is -46.11 kJ/mol, the reaction is exergonic.
Chemical thermodynamics, exergonic reactions are chemical reactions in which the change in free energy is negative (there is a net release of free energy).
This shows the spontaneous reaction when the system is closed and the initial and final temperatures are the same.
Reactions can be endergonic or exergonic. Endergonic reactions require heat or energy input. The exergonic reaction is the opposite. it gives off heat. Answer and Explanation:
Reactions with negative values of G are exergonic, meaning they release heat.
Read more about endothermic.;
https://brainly.com/question/15539215
#SPJ4
In PbO + 2HCl → PbCl2 + H20,13 g of lead oxide is
mixed with 6.4g of hydrochloric acid to produce lead chloride
and water. Which of the following is true?
Answers
Answer:
HCl acts as an excess reagent
Explanation:
The reaction equation is;
PbO + 2HCl → PbCl2 + H20
Number of moles of PbO = 13g/223.2 g/mol = 0.058 moles
Since the mole ratio is 1:1, 0.058 moles of PbCl2 is produced
Number of moles of HCl = 6.4 g/36.5g/mol = 0.175 moles
2 moles of HCl yields 1 mole of PbCl2
0.175 moles yields 0.175 moles * 1/2
= 0.0875 moles of PbCl2
Hence; PbO yields the least number of moles of product so it is the limiting reactant and HCl is the reactant in excess
what is not a colligative property
Answers
Qualities of a solution known as coagulative qualities rely on the quantity of solute particles present but not on the kind of solute.
Boiling point elevation, osmotic pressure, and vapour pressure depression are a few examples of colligative qualities. Solubility is the response to the query of what is not a collative property.
The amount of a solute that can dissolve in a solvent is known as its solubility, and the solute's type does affect this quantity. Solubility is not a collative quality, then.
Learn more about colligative property at:
https://brainly.com/question/30799665
#SPJ1
Which of the following statements is NOT
true about isotopes of the same element?
1. They always have different atomic
weights.
2. They always have different numbers of
protons.
3. They always have the same atomic number.
4. They always have different numbers of neutrons.
Answers
Answer:
answer is 2 ............
The water table is defined as?
a) Pumping level in a well
b) Upper surface of the groundwater
c) Water level in a reservoir
d) Water level obtained in a well after penetrating several aquifers
Answers
The water table is defined as: Option b) Upper surface of the groundwater
The water table is an underground line separating the soil's surface from the region where groundwater seeps into rock crevices and voids between sediments. At this limit, the water pressure and atmospheric pressure are equal.
The unsaturated zone is the portion of the soil surface above the water table where water and oxygen coexist in the gaps between the sediments. Because there is oxygen in the soil, the unsaturated zone is also known as the zone of aeration. The saturated zone, when water completely fills the crevices between the sediments, is located beneath the water table. Impenetrable rock surrounds the saturated zone at its base.
To know more about water table click here:
https://brainly.com/question/30192753
#SPJ11
When a Lewis acid and a Lewis base combine, the product may be referred to as : None of the options.
Adduct Bronsted Base Anode Bronsted Acid
Answers
When a Lewis acid and a Lewis base combine, the product may be referred to as an adduct.
An adduct is a chemical species that is formed when a Lewis acid and a Lewis base combine through a coordinate covalent bond. In this reaction, the Lewis base donates a pair of electrons to the Lewis acid, which accepts the electrons. This results in the formation of a new molecule, called an adduct, which contains a new covalent bond.
An example of an adduct is the reaction between boron trifluoride (BF3) and ammonia (NH3) to form boron trifluoride ammonia complex (BF3•NH3), which is an adduct.
To know more about Lewis acid,
https://brainly.com/question/15220646
#SPJ11
Isobutane (C4H10) is one of the components of natural gas. Which equation
shows the balanced combustion reaction for isobutane?
A. C4H10 + O2 + 4CO2 + H2O + heat
B. C4H10 + O2 + CO2 + H2O + heat
C. C4H10 + O2 + 4C02 + 5H20 + heat
D. C4H10 +6.502 → 4002 + 5H20 + heat
SUBMIT

Answers
Answer:
D. C₄H₁₀ + 6.5O₂ → 4CO₂ + 5H₂O + heat
Explanation:
The reaction of the combustion of isobutane with oxygen is presented as follows;
C₄H₁₀ + 6.5O₂ → 4CO₂ + 5H₂O + heat
In the above reaction, the number of elements on the reactant side are;
4 carbon, C, atoms, 10 hydrogen, H, atoms, and 13 oxygen, O, atoms
The number of elements on the product side of the above chemical reaction are;
The number of carbon atoms, C = 4
The number of hydrogen atoms, H = 10
The number of oxygen atoms, O = 13
Therefore, the number of atoms on the reactant side of the chemical equation are equal to the number of atoms on the product side, and the combustion reaction for isobutane is balanced.
A car is accelerating from rest at a rate of 2 (m/s)/ s. How fast will it be going after 4 seconds?
Answers
Answer:
8 m/s
Explanation:
The Formula
v = u + at⇒ v = final velocity
⇒ u = initial velocity
⇒ a = acceleration
⇒ t = time taken
Given
u = 0 [rest]a = 2 m/st = 4 sSolving
v = u + atv = 0 + 2(4)v = 8 m/sthe graph above describes the location of an electron in a hydrogen atom that is in the ground state. what conclusion can be drawn from the graph?
Answers
By analyzing the graph the conclusion that can be derived about the location of an electron in a hydrogen atom that is in the ground state is that the greatest probability of locating electron is at a distance of one Bohr radius from the nucleus.
Generally the Bohr radius is described as a physical constant that is used to represent the most probable distance between the electron and nucleus of a hydrogen atom at its ground state (which is the lowest energy level). The constant's value of Bohr radius is symbolized as a₀, and its value is approximately 5.29177210903(80) x 10⁻¹¹ meters (m).
Hence, the greatest probability of locating electron is at a distance of one Bohr radius from the nucleus.
The graph is given is the image attached below.
Learn more about Bohr radius from the link given below.
https://brainly.com/question/31131977
#SPJ1

if a sample of chloroform is initially 25 degrees celsius what is the final temperature if 150.0g of chloroform absorbs 1.0 kilojoules of heat, and the specific heat of chloroform is 0.96 J/g degrees celsius
Answers
Taking into account the definition of sensible heat, the final temperature if 150.0g of chloroform absorbs 1.0 kilojoules of heat is 31.94 C.
Definition of sensible heatWhen heat added or removed from a substance causes a temperature change in it without affecting its physical state (phase change), it is called sensible heat.
The expression that allows to calculate heat exchanges is:
Q = c× m× ΔT
where:
Q is the heat exchanged by a body of mass m.c is the specific heat substance.ΔT is the temperature variation.Specific heat of the substanceFinal temperatureIn this case, you know:
Q= 1 kJ= 1000 Jc= 0.96 J/gCm= 150 gΔT= Tfinal - Tinitial= Tfinal - 25 CReplacing in the definition of sensible heat:
1000 J = 0.96 J/gC× 150 g× (Tfinal - 25 C)
Solving:
1000 J = 144 J/C× (Tfinal - 25 C)
1000 J÷ 144 J/C= Tfinal - 25 C
6.94 C= Tfinal - 25 C
6.94 C + 25 C= Tfinal
31.94 C= Tfinal
Finally, the final temperature is 31.94 C.
Learn more about sensible heat:
brainly.com/question/12670283
#SPJ1
EMERGENCY HELP NEEDED!! WILL MARK BRAINLIEST!!!
Study the table of an object’s motion.



Answers
To determine the velocity of a moving body using a graph, you need to look at the slope of the graph. The slope of a position vs time graph gives you the velocity of the object.
What are the steps to be followed to determine the velocity of a moving body using a graph?Plot the position of the object on the y-axis and time on the x-axis.Draw a tangent to the curve at any point on the graph.Find the slope of the tangent line. You can do this by dividing the change in position by the change in time between two points on the tangent line.Repeat steps 2 and 3 for several points on the graph.The average of all the slopes you calculated will give you the average velocity of the object over the time interval you selected.Alternatively, you can use a velocity vs time graph to determine the velocity of a moving body. In this case, the slope of the graph gives you the acceleration of the object.
To know more about velocity, visit:
https://brainly.com/question/17127206
#SPJ1
A mixture of gases collected over water at 14∘ C has a total pressure of 1.198 atm and occupies 72 mL. How many grams of water escaped into the vapour phase?
Answers
Approximately 0.00686 grams of water escaped into the vapor phase.
To solve this problem, we need to use the concept of partial pressure. The total pressure of the gas mixture is the sum of the partial pressures of each gas in the mixture. In this case, we have a mixture of gases collected over water, so the partial pressure of water vapor is also a factor.
First, we need to calculate the partial pressure of water vapor. At 14∘C, the vapor pressure of water is 12.76 mmHg or 0.0167 atm. This means that the partial pressure of water vapor in the gas mixture is 0.0167 atm.
To find out how much water escaped into the vapor phase, we can use the following equation:
n = PV/RT
where n is the number of moles of water vapor, P is the partial pressure of water vapor, V is the volume of the gas mixture, R is the gas constant (0.0821 L atm/mol K), and T is the temperature in Kelvin (287 K).
Plugging in the values, we get:
n = (0.0167 atm)(0.072 L)/(0.0821 L atm/mol K)(287 K) = 0.000381 mol
Now, we need to convert moles to grams. The molar mass of water is 18.015 g/mol. So:
mass = n x molar mass = 0.000381 mol x 18.015 g/mol = 0.00686 g
Therefore, approximately 0.00686 grams of water escaped into the vapor phase.
Learn more about vapor phase here
https://brainly.com/question/2400738
#SPJ11
Need help on this question asap pleasee
No links

Answers
Answer:
Endothermic
Explanation:
An endothermic reaction is one in which energy is absorbed while an exothermic reaction is one in which energy is given out(evolved).
If we look at the reaction as written; 3C(graphite) + 3H2(g) + 88kcal -------> C3H8(g), we will notice that;
i) 88kcal of energy had to be absorbed for the reaction to occur
ii) The enthalpy change for the reaction is positive.
Since energy must be taken in to drive the forward reaction, then the reaction is endothermic as written.
What is evaporation
Answers
Answer:
The process of turning from liquid into vapor.
Explanation:
What is the % of each element in Ni3{PO4) 2 ?
Answers
Answer:
nickel 48.1063%
Phosphorus 16.9245%
Oxygen 34.9692%
The percent composition of each element like nitrogen, oxygen and phosphorous in Ni₃(PO₄)₂ are 48.03%, 34.97% and 16.93% respectively.
How do we calculate % composition?Percent composition of any element present in any compound will be calculated as:
% comosition = (Mass of element / Mass of compound)×100%
Mass of Ni₃(PO₄)₂ compound = 366.02 g/mol
Molar mass of 3 Nitrogen atoms = 3×58.6 = 175.8 g/mol
Moar mass of 2 Phosphorous atoms = 2×31 = 62 g/mol
Moar mass of 8 Oxygen atoms = 8×16 = 128 g/mol
% comosition of Nitrogen = (175.8/366.02)×100% = 48.03%
% comosition of Oxygen = (128/366.02)×100% = 34.97%
% comosition of Phosphorous = (62/366.02)×100% = 16.93%
Hence % composition of nitrogen, oxygen and phosphorous is 48.03%, 34.97% and 16.93% respectively.
To know more about percent composition, visit the below link:
https://brainly.com/question/21044245
He amount of the forward and reverse primers they ordered is 234 μg and 216
ug respectively. The molar mass of the forward primer is 6500 g/mol and that of
the reverse primer is 6100 g/mol. They need to prepare a 100 μM solutions of
both forward and reverse primers. Assuming that the volume contributed by the
forward and reverse primer is equal to zero (i. E. , volume of solute = 0, therefore
the volume of water is equal to volume of solution), calculate the volume of water
in which they need to dissolve the forward and reverse primers
Answers
The volume of the DNA solution, is 4μL, that needs to be added to the reaction mixture.
What is Dilution Equation?The Dilution Equation is represented as
M₁V₁ = M₂V₂
where,
M₁ represent the Molarity of Stock solution
M₂ represent the Molarity of new solution
V₁ represent the initial volume from stock solution
V₂ represent the final volume from stock solution
Here
M₁ = 12.5 ng/μL
M₂ = 1 ng/μL
V₂ = 50 μL
Now put the values in above formula we get the initial volume as
M₁V₁ = M₂V₂
(12.5 ng/μL) x V₁ = (1 ng/μL) x 50 μL
12.5 x V₁ = 1 x 50 μL
V₁ = 50 / 12.5 μL
V₁ = 4 μL
Learn more about the Dilution Equation here: https://brainly.com/question/24709069
#SPJ4
NOTE: The given question is incomplete on the portal. Here is the complete question.
QUESTION: Let's say that you ordered 2.00 μg of double stranded DNA. You want to conduct an experiment but you need to prepare a DNA solution whose concentration is 12.5 ng DNA/1 μL of water. You then ordered forward (the amount being 234 μg) and reverse primers (the amount being 216 ug). You figure out that the molar mass of the forward primer is 6500 g/mol and that of the reverse primer is 6100 g/mol. You need to prepare a 100 μM solutions of both forward and reverse primers.
You then received an enzyme whose concentration is 5 units/μL. You also received a buffer that has a 200 mM Tris-HCl (pH 8.4), 500 mM KCl. They call this a 10X buffer (10 times the working concentration of the buffer). Working concentration is the concentration of Buffer when the PCR reaction is setup. Using all these solutions they plan to setup a new PCR reaction with a final reaction volume of 50 μL.
The final concentration of the DNA in the reaction mixture is 1 ng/ μL. Calculate the volume of the DNA solution (that was prepared earlier), in μL, that needs to be added to reaction mixture.
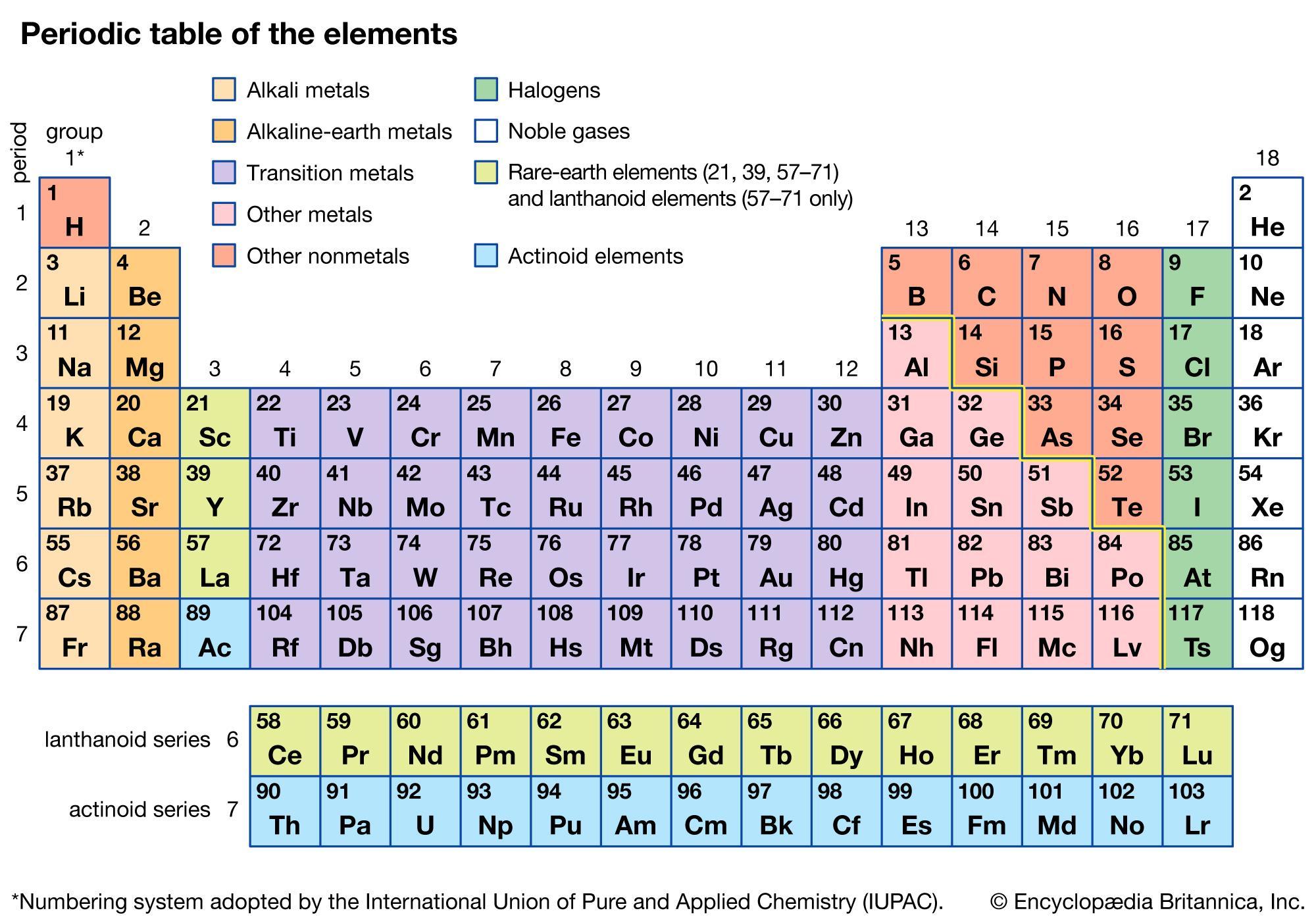
Which elements are both classified as metalloids?
Ge and As
Bi and Po
B and C
Si and P
Answers
they are lined up between the non metals and metals so they are metalloids
Water 3.0 deals mainly with sewage treatment.
Describe which chemicals are currently not broken down by currently
used wastewater technologies and why that is important.
Answers
Water 3.0 deals mainly with sewage treatment. The primary aim of this project is to reduce the harmful impacts of chemical pollutants from industrial and agricultural activities on natural water resources.
Currently, used wastewater treatment technologies can break down some of the chemicals in wastewater but not all of them. Chemicals that are not broken down are referred to as persistent organic pollutants. These chemicals persist in the environment for long periods, and they can cause severe damage to aquatic life and human health.
Currently, the primary challenge facing water treatment technologies is the removal of persistent organic pollutants such as pesticides, pharmaceuticals, and endocrine-disrupting chemicals from wastewater.
These pollutants are generally water-soluble and resist microbial degradation, making them hard to remove from wastewater using current water treatment technologies. For example, conventional activated sludge treatment used in wastewater treatment plants does not remove some persistent organic pollutants from wastewater.
Failure to remove these pollutants from wastewater can have significant environmental and health impacts.
For example, pharmaceutical chemicals can cause antibiotic resistance, while endocrine-disrupting chemicals can cause birth defects, cancer, and other health problems.
Therefore, there is a need to improve wastewater treatment technologies to remove persistent organic pollutants from wastewater.
In conclusion, wastewater treatment technologies can break down some chemicals but not all. Chemicals that are not broken down are persistent organic pollutants and pose a significant risk to the environment and human health. Therefore, it is important to develop wastewater treatment technologies that can remove these pollutants from wastewater.
To know more about chemicals visit:
https://brainly.com/question/29240183
#SPJ11
Using the table of average bond energies, estimate the energy needed to break the bonds of the reactants and the energy released when the products from for the reaction N2 + O2 –> 2NO. Note N2 has a triple bond and O2 and NO have double bonds.

Answers
The energy that is released for the breaking of bonds is 229 kJ/mol.
What is the energy released?We should be able to recall that the enthalpy of the reaction taken to be the energy that is evolved or absorbed in the reaction that is ongoing. We have to note that in the course of the reaction there would be the breaking and the making of bonds.
Now we know that;
The bond energy can be given as;
Sum energy of the broken bonds of reactants - Sum of the energy of the formed bonds
Hence;
(945 + 498) - 2(607)
1443 - 1214
= 229 kJ/mol
Learn more about enthalpy:https://brainly.com/question/13996238
#SPJ1
What mass of sucrose needs to be dissolved into water in order to prepare a 15% by mass solution with a total mass of 650 g
Answers

The mass of sucrose needs to be dissolved will be "97.5 g".
SucroseA cube of sugar or sweetener that is composed of yet another molecule of glucose as well as one component of fructose linked simultaneously.
According to the question,
Mass percentage = 15%
Total mass = 650 g
Let,
The mass of sucrose be "x".
Now,
The mass of sucrose will be:
→ 0.15 = \(\frac{x}{650}\)
By applying cross-multiplication, we get
x = 0.15 × 650
= 97.5 g
Thus the above answer is appropriate.
Find out more information about sucrose here:
https://brainly.com/question/211758
Look closely at the Sodium square below the table. Using the information in the square and the position of Sodium on the periodic table, describe sodium atoms with as much detail as you can. Below is a word bank. These are words that can help you with your description. You do not have to use all of them, but you should use some of them. DO NOT just define the words, use the words in your description. Make sure you are writing your OWN words. No copying and pasting. Word bank: protons, neutrons, electrons, atomic mass, atomic number, valence electrons, energy levels, group, period, metal, non-metal, metalloid
Answers
What is your estimate of the strength for each clay type at 50% water content, with DI water as its pore fluid, and with brine in its pore fluid? - Is there a significant difference? If so, what physical mechanism do you think is causing the change in strength? What is the effect of salt on the shear strength of clays?
Answers
The physical mechanism causing the change in strength when using brine as the pore fluid is the presence of salt ions that weaken the interparticle bonds. Salt can reduce the shear strength of clays by increasing the repulsive forces between clay particles.
The strength of clay types at 50% water content can vary depending on whether DI water or brine is used as the pore fluid. Generally, there is a significant difference in strength between the two.
The presence of salt in brine can have an effect on the shear strength of clays. When salt is dissolved in water, it creates ions that can interact with the clay particles. These interactions can lead to the formation of electrical double layers around the clay particles, which can increase the interparticle repulsion and decrease the shear strength of the clay.
On the other hand, when DI water is used as the pore fluid, there is no presence of salt ions to affect the interparticle interactions. As a result, the clay particles can have stronger bonds and higher shear strength compared to when brine is present.
Learn more about interparticle bonds here :-
https://brainly.com/question/4339576
#SPJ11
Convert 3828 ml to L
Answers
Answer:
divide the volume value by 1000
So 3828/1000=3.828
3. Which two of the following elements would you expect to have a very different melting point than titanium (TI): iron (Fe),
sulfur (S), selenium (Se), or chromium (Cr)? Explain why you chose those two elements.
Answers
The two elements that would have a very different melting point than titanium (Ti) are Sulfur (S) and Selenium (Se)
Periodic tableFrom the question, we are to determine the elements that would have different melting point than titanium.
Titanium (Ti) is a transition metal which belongs to 3d- block on the periodic table.
Iron (Fe) and Chromium are also transition metals; and they belong to the 3d- block on the periodic table. Transition metals have high melting points.
Sulfur (S) and Selenium (Se) are Group 16 elements, which will have lower melting points compared to the transition elements.
Hence, the two elements that would have a very different melting point than titanium (Ti) are Sulfur (S) and Selenium (Se)
Learn more on Periodic table here: https://brainly.com/question/13555712
4. Why is it important to neutralize an acid spill
before attempting to clean it up?
Answers
Answer:
It the acid easier to handel
nonenzymatic e1 reactions can often result in a mixture of more than one alkene product. elimination of 'hx' from the following starting compound, for example, could yield three different possible alkene products, true or false?
Answers
The given statement is true that nonenzymatic E1 reactions can often result in a mixture of more than one alkene product. This is due to the presence of different possible elimination products.
Nonenzymatic E1 reactions: E1 is a chemical reaction mechanism that includes the elimination of a leaving group (such as HX) from an organic molecule to create a double bond or alkene. This is a two-step process in which the first step is the formation of a carbocation intermediate.The nonenzymatic E1 reactions can often result in a mixture of more than one alkene product because the carbocation intermediate that forms can be attacked by nucleophiles in various directions, leading to the formation of different elimination products. The regiochemistry of the reaction is determined by the most stable carbocation intermediate formed from the initial step of the reaction.In this case, elimination of HX from the given starting compound can yield three different possible alkene products due to the presence of three different hydrogen atoms that can eliminate. Hence, the given statement is true.Learn more about E1 reactions: https://brainly.com/question/30887510
#SPJ11
1. How many atoms are in 3.5 moles of nickel?
Answers
The number is 6.022 x 10^23 atoms/mol.
MARKING BRAINLIEST :)
Which property has a trend similar to that of electronegativity?
A. First ionization energy
B. Atomic radius
C. Ionic radius
D. Atomic mass
Answers
Answer:
a
Explanation:
There are various kind of elements that are present in periodic table. Some elements are harmful, some are radioactive, some are noble gases. Therefore, the correct option is option A.
What is periodic table?Periodic table is a table in which we find elements with properties like metals, non metals, metalloids and radioactive element arranges in increasing atomic number.
Periodic table help a scientist to know what are the different types of elements are present in periodic table so that they can discover the new elements that are not being discovered yet.
Along period, electronegativity increases. along group electronegativity decreases. The same trend is followed by first ionization energy in periodic table.
Therefore, the correct option is option A.
Learn more about periodic table, here:
https://brainly.com/question/11155928
#SPJ2
Zinc Sulfide reacts with oxygen according to the reaction:
2ZnS (s) + 3 O2(g) -> 2 ZnO (s) + 2 SO2 (g)
A reaction mixture contains 4.2 moles of zinc sulfide and 6.8 moles of oxygen. Once the reaction occurred as completely as possible, what amount in moles is left of the excess reactant?
Answers
The amount in moles of the excess reactant left is 0.5 mole.
Balanced equation- 2ZnS (s) + 3O₂(g) --> 2ZnO (s) + 2SO₂(g).
What is another name for zinc sulfide?
A typical pigment known as sachtolith is zinc sulphide.From the balanced equation,
2 moles of ZnS reacted with 3 moles of O₂
How to determine the excess reactant
From the balanced equation,
2 moles of ZnS reacted with 3 moles of O₂
Therefore,
4.2 moles of ZnS will react with =(4.2 × 3) / 2 = 6.3 moles of O₂
From the calculations made above, we can see that only 6.3 moles of O₂ out of 6.8 moles given, is required to react completely with 4.2 moles of ZnS.
Thus, ZnS is the limiting reactant and O₂ is the excess reactant.
How to determine the mole of the excess reactant remaining
The excess reactant is O₂. Thus the mole remaining after the reaction can be obtained as illustrated below:
Mole of O₂ given = 6.8 moles
Mole of O₂ that reacted = 6.3 moles
Mole of O₂ remaining =?
Mole of O₂ remaining = (Mole of O₂ given) - (Mole of O₂ that reacted)
Mole of O₂ remaining = 6.8 - 6.3
Mole of O₂ remaining = 0.5 mole
To learn more about reactant refer to:
https://brainly.com/question/25685654
#SPJ1