true/false. propose a synthesis starting with ethanol and ethyl butanoate
Answers
True/false questions require a statement to be either true or false, but the given prompt is incomplete and does not form a statement. Therefore, it cannot be classified as true or false.
However, to provide some clarification, the prompt seems to be asking for a synthesis pathway starting with ethanol and ethyl butanoate. Ethanol (\(C_2H_5OH\)) and ethyl butanoate (\(C_6H_{12}O_2\)) are organic compounds that can be used in organic synthesis to produce other chemicals. One possible pathway for the synthesis of a compound starting with these two compounds is:
Convert ethanol to ethene (\(C_2H_4\)) by dehydrating it with a strong acid such as sulfuric acid.
React ethene with hydrogen (\(H_2\)) using a nickel catalyst to produce ethane (\(C_2H_6\)).React ethane with chlorine (\(C_{l2}\)) using ultraviolet light to produce chloroethane (\(C_2H_5Cl\)).React chloroethane with sodium butanoate (\(C_4H_7NaO_2\)) in the presence of sodium hydroxide (NaOH) to produce ethyl butanoate (\(C_6H_{12}O_2\)) and sodium chloride (NaCl).This synthesis pathway is just one example, and there can be multiple ways to synthesize a compound starting with different starting materials.
Learn more about Synthesis pathway at
brainly.com/question/16013771
#SPJ1
Related Questions
the chemical reaction in which light is absorbed in the photoreceptor causing the chemical rhodopsin to separate between retinal and opsin is known as
Answers
The phototransduction reaction is the process by which opsin and retinal are separated chemically by rhodopsin.
What is rhodopsin?
Rhodopsin, as well referred to as visual purple, is a G-protein-coupled receptor and a protein that is encoded by the RHO gene (GPCR). The retinal rod cells' opsin, a protein light-sensitive receptor, is what initiates visual phototransduction throughout rods. Rhodopsin is incredibly sensitive to light because it mediates vision in low light. Rhodopsin begins to photobleach as soon as it is exposed to light. In humans, it takes around 30 minutes to fully regenerate, at which point the rods become more sensitive. Eye conditions like retinitis pigmentosa as well as rare genetic stationary night blindness are brought on by rhodopsin gene defects.
This reaction occurs in the photoreceptor cells of the retina and involves the absorption of light by the photopigment rhodopsin. When light is absorbed, it causes a conformational change in rhodopsin, which in turn causes the molecule to separate into two parts—retinal and opsin. The separated retinal and opsin molecules then trigger a cascade of biochemical reactions that lead to depolarization of the photoreceptor cell and the release of neurotransmitters, which in turn activate the cells of the optic nerve.
To learn more about rhodopsin
https://brainly.com/question/3648231
#SPJ4
I understand that I answered wrong with 15ml instead of 14, but would I have to put the 0 after or no?

Answers
Answer:
no dont worry its in decimal point
anything after the point is considered as not to the fullest
bromine water can be used to distinguish between these two molecules?? true or false
Answers
Answer:i dkd
Explanation:
jsach
In the Millikan oil droplet experiment, the oil is sprayed from an atomizer into a chamber. The droplets are allowed to pass through the hole into the chamber so that their fall can be observed. The top and bottom of the chamber consist of electrically charged plates. The upper plate is positively charged, and the lower plate is negatively charged. X rays are introduced into the chamber so that when they strike the oil droplets, the droplets will acquire one or more negative charges. The electric field (voltage) is applied to the metal plates.
Watch the animation and identify the effects of an electric field on the motion of a negatively charged oil droplet. Consider the gravitational force as Fg and the electric force as Fe. All the other forces acting on the oil droplet can be ignored as their effect on the motion of the oil droplet is negligible.
A/ In the absence of an electric field, the oil droplet falls freely due to the gravitational force.
B/ If Fe is increased until it is equal to Fg, the negatively charged oil droplet will remain stationary.
C/ If Fe is greater than Fg, the negatively charged oil droplet will move freely toward the negatively charged plate.
D/ In the presence of an electric field, the negatively charged oil droplet moves freely toward the negatively charged plate.
** I chose B, but that was the wrong answer
Answers
C/ If Fe is greater than Fg, the negatively charged oil droplet will move freely toward the negatively charged plate.
In the Millikan oil droplet experiment, the negatively charged oil droplets are subjected to an electric field created by the charged plates. The electric force (Fe) acts on the oil droplet in a direction opposite to the gravitational force (Fg). When Fe is greater than Fg, the electric force overcomes the gravitational force, causing the negatively charged oil droplet to experience an upward force. As a result, the oil droplet moves freely upward toward the negatively charged plate.
Option B is incorrect because if Fe is equal to Fg, the forces balance each other, resulting in a stationary droplet. However, the question states that Fe is increased until it is greater than Fg, implying that the droplet is no longer stationary but moves in response to the electric force.
Therefore, option C is the correct answer, as it describes the effect of an electric field on the motion of a negatively charged oil droplet in the Millikan oil droplet experiment.
To learn more about Millikan oil droplet experiment, here
https://brainly.com/question/32330429
#SPJ4
A. Lithium and cadmium are in the mixture. Strontium is not in the mixture.B. Lithium and strontium are in the mixture. Cadmium is not in the mixture.C. Cadmium and strontium are in the mixture. Lithium is not in the mixture.D. All of the shown elements are present in the mixture.

Answers
Answer:
I think it's Sr and Li...
Explanation:
You just line up the elements to the mixture and whatever fits with all of the charts are the elements present in the mixture.
At 50 degree C the value of KW is 5.5 * 10-14. What is the concentration of H3O+ in a neutral solution at 50 degree C?
Answers
To find the concentration of H3O+ in a neutral solution at 50 degrees C, we can use the information given about the value of KW, which is 5.5 * 10^-14 at that temperature.
In a neutral solution, the concentration of H3O+ ions is equal to the concentration of OH- ions. We can use the relationship between KW, H3O+, and OH- ions to solve for the concentration:
KW = [H3O+] * [OH-]
Since it's a neutral solution, [H3O+] = [OH-], so we can write:
KW = [H3O+]^2
Now, we can solve for the concentration of H3O+ ions:
5.5 * 10^-14 = [H3O+]^2
To find the concentration of H3O+ ions, we take the square root of both sides:
[H3O+] = sqrt(5.5 * 10^-14)
[H3O+] ≈ 7.4 * 10^-8 M
So, the concentration of H3O+ in a neutral solution at 50 degrees C is approximately 7.4 * 10^-8 M.
https://brainly.com/question/12920721
#SPJ11
Which solute, an electrolyte or a nonelectrolyte, has the greater effect on the boiling point when a given amount of each
solute is dissolved in the same mass of water?
O The nonelectrolyte does because it disperses into molecules.
O The electrolyte does because it disperses into molecules.
O The nonelectrolyte does because it dissociates into ions.
O The electrolyte does because it dissociates into ions.
Answers
The solute wether electrolyte or a nonelectrolyte, has the greater effect on the boiling point when a given amount of each solute is dissolved in the same mass of water The nonelectrolyte does because it dissociates into ions.
What is a solute?Solute refers to substances or liquid which can readily dissolve in a solvent. It's concentration is lower to that of solvent.
Therefore, The solute wether electrolyte or a nonelectrolyte, has the greater effect on the boiling point when a given amount of each solute is dissolved in the same mass of water The nonelectrolyte does because it dissociates into ions
Learn more about solute below.
https://brainly.com/question/16083884
#SPJ2
Answer:
D: The electrolyte does because it dissociates into ions.
Explanation:
Name two inputs, two outputs and two side effects of the healthcare system.
Answers
Answer:
Healthcare system is one of the most important in any country’s economy.
Health care system Inputs include Medication, patients, locations
Outputs: Treating injured or sick people, taking care of people's health
Side effects: Lack of medication/drugs or no right medication, wrong diagnosis or prescription.
Nuclear fission occurs when _______________ a. TNT and plutonium are combined, causing the molecules to separate. b. a nucleus breaks up into two equal fragments that release and separate more atoms. c. like atoms collide to create double nuclei. d. trinitite is created by multiple molecules that form a single atom.
Answers
Nuclear fission occurs when a nucleus breaks up into two equal fragments that release and separate more atoms. So, the correct option is B.
Nuclear fission is a process in which the nucleus of an atom breaks apart into two or more smaller nuclei. This process releases a significant amount of energy.
Option B accurately describes the process of nuclear fission. When a heavy nucleus, such as uranium-235 or plutonium-239, absorbs a neutron, it becomes unstable and splits into two smaller nuclei.These smaller nuclei, along with additional neutrons, are released in the process. The release of neutrons can trigger a chain reaction, where each neutron released can potentially collide with other nuclei, causing them to undergo fission as well.The energy released during nuclear fission is due to the conversion of a small amount of mass into a large amount of energy, as described by Einstein's famous equation, E=mc².This energy is utilized in various applications, including nuclear power generation and nuclear weapons. Nuclear fission reactions are carefully controlled in nuclear power plants to ensure the sustained release of energy without leading to uncontrolled chain reactions. Hence the correct option is B.
For more questions on nucleus
https://brainly.com/question/29855834
#SPJ8
The castaways have been on an island for 15 days. They have created a game to pass the time. Each castaway throws a coconut and whoever throws theirs with the highest average speed wins. How many seconds did it take for the third coconut to travel 30 m?
Answers
The answer would be 2.
Basically all you do is t = d/s which means time equals distance divided by speed. And your answer will be 2
Determine the amount of iron in a dietary supplement, a random sample of 15 tablets weighing a total of 23. 58 g was ground into a fine powder. A 3. 1289 -g sample of the powdered tablets was dissolved and treated to precipitate the iron as Fe(OH)3. The precipitate was collected, rinsed, and ignited to constant weight as Fe2O3, yielding 0. 3691 g. Calculate the iron content of the dietary supplement as g FeSO4. 7H2O per tablet
Answers
In order to calculate the iron content of the dietary supplement as g FeSO4. 7H2O per tablet, we need to do the following steps: Calculate the weight of the 15 tablets that were ground into a fine powder Divide the weight by 15 to find the weight of one tablet .
Calculate the number of moles of Fe2O3 Calculate the number of moles of FeCalculate the weight of Fe in one tabletCalculate the weight of FeSO4.7H2O per tablet by multiplying by the appropriate factorCalculation:Weight of the 15 tablets = 23.58 gWeight of one tablet = 23.58 g/15 = 1.572 gThe mass of Fe2O3 = 0.3691 gThe molar mass of Fe2O3 = 159.69 g/molThe number of moles of Fe2O3 can be calculated as:Number of moles of Fe2O3 = mass/molar mass= 0.3691 g/159.69 g/mol= 0.002315 mol The balanced equation for the reaction of Fe(OH)3 to Fe2O3 is:2Fe(OH)3 → Fe2O3 + 3H2O.
From this equation, we can see that 2 moles of Fe(OH)3 produce 1 mole of Fe2O3. Therefore, the number of moles of Fe in the sample is: Number of moles of Fe = 0.002315 mol/2 = 0.0011575 molThe molar mass of Fe is 55.85 g/mol. The mass of Fe in one tablet can be calculated as:Mass of Fe in one tablet = number of moles x molar mass= 0.0011575 mol x 55.85 g/mol= 0.06467 g or 64.67 mgTo calculate the weight of FeSO4.7H2O per tablet, we need to multiply the mass of Fe per tablet by the appropriate factor.The molecular weight of FeSO4.7H2O = (55.85 + 32.07 + 4*16.00 + 7*18.02) g/mol = 278.01 g/molThe molecular weight of Fe in FeSO4.7H2O = 55.85 g/molThe mass of FeSO4.7H2O per tablet can be calculated as:Mass of FeSO4.7H2O per tablet = (64.67 mg Fe/tablet) x (1 tablet/1000 mg) x (1 mol FeSO4.7H2O/1 mol Fe) x (278.01 g/mol FeSO4.7H2O)= 0.0180 g FeSO4.7H2O per tabletTherefore, the iron content of the dietary supplement as g FeSO4.7H2O per tablet is 0.0180 g FeSO4.7H2O per tablet.
To know more about Calculate visit :
https://brainly.com/question/29240183
#SPJ11
The iron content of the dietary supplement is approximately 0.0246 g of \(FeSO_4.7H_2O\) per tablet.
1. Calculate the mass of the iron precipitate (\(Fe_2O_3\)):
Mass of \(Fe_2O_3\) = Mass of ignited precipitate - Mass of ignited empty crucible
= 0.3691 g - 0 g (assuming the crucible was empty before ignition)
= 0.3691 g
2. Convert the mass of \(Fe_2O_3\) to moles of \(Fe_2O_3\):
Molar mass of \(Fe_2O_3\) = 159.69 g/mol (atomic masses: Fe = 55.845 g/mol, O = 16 g/mol)
Moles of \(Fe_2O_3\) = Mass of \(Fe_2O_3\) / Molar mass of \(Fe_2O_3\)
= 0.3691 g / 159.69 g/mol
= 0.002313 mol
3. Determine the moles of Fe in \(Fe_2O_3\):
Since the molar ratio of Fe to \(Fe_2O_3\) is 2:3, the moles of Fe can be calculated as:
Moles of Fe = (2/3) * Moles of \(Fe_2O_3\)
= (2/3) * 0.002313 mol
= 0.001542 mol
4. Calculate the molar mass of Fe:
Molar mass of Fe = 55.845 g/mol
5. Find the mass of Fe in grams:
Mass of Fe = Moles of Fe * Molar mass of Fe
= 0.001542 mol * 55.845 g/mol
= 0.08616 g
6. Determine the number of moles of \(FeSO_4.7H_2O\):
From the balanced chemical equation: \(Fe_2O_3\) + \(3H_2SO_4\) -> \(Fe_2\)(\(SO_4\))3 + \(3H_2O\)
The stoichiometric ratio shows that 1 mole of \(Fe_2O_3\) reacts with 3 moles of \(H_2SO_4\).
Therefore, 1 mole of \(Fe_2O_3\) corresponds to 1/3 moles of \(FeSO_4.7H_2O\).
Moles of \(FeSO_4.7H_2O\) = (1/3) * Moles of \(Fe_2O_3\)
= (1/3) * 0.002313 mol
= 0.000771 mol
7. Calculate the mass of \(FeSO_4.7H_2O\):
Molar mass of \(FeSO_4.7H_2O\) = 278.01 g/mol (atomic masses: Fe = 55.845 g/mol, S = 32.06 g/mol, O = 16 g/mol, H = 1 g/mol)
Mass of \(FeSO_4.7H_2O\) = Moles of \(FeSO_4.7H_2O\) * Molar mass of \(FeSO_4.7H_2O\)
= 0.000771 mol * 278.01 g/mol
= 0.2141 g
8. Calculate the iron content per tablet:
Iron content per tablet = Mass of \(FeSO_4.7H_2O\) / Number of tablets
= 0.2141 g / 15 tablets
≈ 0.01427 g
Therefore, the iron content of the dietary supplement is approximately 0.01427 g of \(FeSO_4.7H_2O\) per tablet.
For more such questions on iron, click on:
https://brainly.com/question/2596300
#SPJ8
A piece of iron is heated to 120'C then placed in a calorimeter containing 50g of water. The water temperature is raised from 30°C to 40°C. Assuming the specific heat of water is 4. 18 J/8'C and the specific heat of
Iron is 0. 44 J/g C, what is the mass of the piece of iron?
118. 8
59. 45
39. 6
752
Answers
Answer:
Mass of iron = 59.375 gm
Explanation:
Calories ( or joules) are added to the water by the hot steel so at the endpoint they are BOTH at 40 C
The water gains:
4.18 j/g-C * 50 * (40-30 C) = 2090 j
The steel gave up 2090 j going from 120 to 40 C
2090 = .44 j/g-C * m * (120-40) solve fro m = 59.375 gm
a 1) How would you make 1 liter of a 10% NaCl solution from a solid stock? Provide details of what kind of containers you would use.
Answers
To make 1 liter of a 10% NaCl solution from a solid stock, you will require the following materials and containers.MaterialsSolid NaClDistilled water1-Liter volumetric flask250-mL volumetric flask 2-beakersProcedureTo prepare 1 liter of a 10% NaCl solution, the following procedure should be followed:Measure out 100g of NaCl using a balance.
Measure the weight of an empty 250-mL volumetric flask.Add the NaCl to a 250-mL beaker and add a small amount of distilled water to it to dissolve the NaCl.Carefully pour the dissolved NaCl solution into the 250-mL volumetric flask. Add distilled water to the mark on the flask to make up the volume. Stopper the flask and invert it several times to mix the solution.Measure the weight of the 1-Liter volumetric flask.Add the 250-mL volumetric flask solution to a 1-Liter volumetric flask.Add distilled water to the mark on the flask to make up the volume.
Stopper the flask and invert it several times to mix the solution.The final volume of the solution will be 1 liter of a 10% NaCl solution.PrecautionsEnsure the NaCl has completely dissolved before adding more water to avoid making a less concentrated solution.Measure the weight of the volumetric flask before and after adding the solution to calculate the volume of solution that was added.Use distilled water to prepare the solution.
To know more about volumetric flask visit:-
https://brainly.com/question/28997155
#SPJ11
according to the video, why should a used chemical container never be refilled? used chemicals may be contaminated, which could cause an unexpected reaction.
Answers
Yes, that is correct. A used chemical container should never be refilled because used chemicals may be contaminated, which could cause an unexpected reaction or even a hazardous situation.
Additionally, used containers may have residue from the previous chemical that could react with the new chemical being added, leading to unexpected and potentially dangerous results. It is always recommended to use a clean and properly labeled container for each chemical to ensure safe storage and use.
A chemical container is a device used to store and transport chemicals and other hazardous materials. It is usually made of materials such as plastic, metal, or glass that are resistant to corrosion and chemical reactions with the contents. Chemical containers are designed to be leak-proof and safe to handle, and are often labeled with warnings and safety information to ensure proper use and handling.
Find more about chemical
brainly.com/question/30155073
#SPJ4
a ground state hydrogen atom absorbs a photon of light having a wavelength of 92.6 nm. what is the final state of the hydrogen atom?
Answers
When a ground state hydrogen atom absorbs a photon of light having a wavelength of 92.6 nm, the final state of the hydrogen atom is the excited state.
The hydrogen atom has only one electron, which is located in the ground state or the first energy level. When a photon of light of 92.6 nm wavelength is absorbed, the electron gains energy and jumps to the higher energy level, which is the second energy level (n = 2).
Thus, the final state of the hydrogen atom is the excited state or the second energy level. The energy absorbed by the electron is equal to the energy of the photon. The energy of a photon is given by the formula: Energy of a photon = hc/λwhere,h = Planck's constant = 6.626 x 10⁻³⁴ J.s
c = speed of light = 3 x 10⁸ m/s λ = wavelength of the photon
Substituting the given values, we get
Energy of a photon = (6.626 x 10⁻³⁴ J.s x 3 x 10⁸ m/s) / (92.6 x 10⁻⁹ m)
Energy of a photon = 2.14 x 10⁻¹⁸ J. The energy absorbed by the electron is equal to the energy difference between the two energy levels.
The energy of an electron in the nth energy level of the hydrogen atom is given by the formula: E_n = (-2.18 x 10⁻¹⁸ J) / n² where, E_n = energy of electron in nth energy level
Substituting n = 1 (ground state), we get: E₁ = (-2.18 x 10⁻¹⁸ J) / (1)² E₁= -2.18 x 10⁻¹⁸ J
Substituting n = 2 (excited state), we get: E₂ = (-2.18 x 10⁻¹⁸ J) / (2)² E₂ = -0.545 x 10⁻¹⁸ J
The energy absorbed by the electron is the difference between the energy of the electron in the excited state and the energy of the electron in the ground state.
ΔE = E₂ - E₁
ΔE = (-0.545 x 10⁻¹⁸ J) - (-2.18 x 10⁻¹⁸ J)ΔE = 1.64 x 10⁻¹⁸ J
Since the electron gains energy, the energy absorbed by the electron is positive. Therefore, the final state of the hydrogen atom is the excited state.
To know more about ground state, refer
https://brainly.com/question/30546051
#SPJ11
what is the atomic number of the atom pictured?

Answers
The atomic number of the atom shown in the picture would be nine because there is a total of nine protons present inside the nucleus of the atom and the number of protons represents the atomic number of the atom.
The number of neutrons is unrelated to the atomic number of an atom.
What is the atomic number?An atom's atomic number is determined by the total number of protons it contains.
The number of neutrons is unrelated to the atomic number of an atom.
The atoms in the image would have an atomic number of nine since its nucleus contains a total of nine protons, and the number of protons corresponds to the atomic number of the atom.
Thus, the atomic number of the atom shown would be nine.
To learn more about the atomic number from here, refer to the link;
brainly.com/question/14190064
#SPJ2
what is the number of moles of sodium in 145g of sodium
Answers
Answer:14.1
Explanation:
Answer:
The number of moles of Sodium in 145g of Sodium is 6.304 moles.
From Periodic Table, we can find that Molecular Weight of Sodium(Na) is 23.
we know the Molar Weight of any element is numerically equal to its Molecular Weight.
So, the number of moles of Sodium in 145g of Sodium = \(\frac{145}{23}\) = 6.304 moles.
Read more about Molar Weight :
https://brainly.com/question/14507491?referrer=searchResults
https://brainly.com/question/14683798?referrer=searchResults
please help me i need this good grade

Answers
What does temperature surround when the kinetic energy is moving fast ?
Answers
When the average kinetic energy of its particles increases, the object's thermal energy increases. Therefore, the thermal energy of an object increases as its temperature increases.
In chemistry, we define the temperature of a substance as the average kinetic energy of all the atoms or molecules of that substance. Not all of the particles of a substance have the same kinetic energy. At any given time, the kinetic energy of the particles can be represented by a distribution.
In physics, temperature is defined as the average kinetic energy of the particles in an object. ... When particles move more quickly, temperature is higher and an object feels warmer. When particles move more slowly, temperature is lower and an object feels cooler.
乁(ᵔヮᵔ)ㄏ
When 60.0 g methane (CH4) is placed in a 1.00-L vessel, the pressure is measured to be 130 atm. Calculate the temperature of the gas using (a) the ideal gas law and (b) the van der Waals equation. Do attractive or repulsive forces dominate
Answers
Answer:
(a) By the ideal gas law, the temperature of the gas is approximately 423.51129 K
(b) By the Van der Waals equation, the temperature of the gas is approximately 442.00558 K
Explanation:
The given parameters are;
The mass of the methane gas, m = 60 g
The volume of the container in which the gas is placed, V = 1.00-L
The pressure of the gas in the container, P = 130 atm
The molar mass of methane, CH₄ = 16.04 g/mol
The Van der Waals Constant for Methane (CH₄) a = 2.253 atm L²·mole⁻² and b = 0.04278 L·mol⁻¹
The universal gas constant, R = 0.08206 L·atm·mol⁻¹·K⁻¹
The number of moles of methane present, n = 60.0 g/(16.04 g/mol) ≈ 3.7406 48 moles
(a) The ideal gas law is P·V = n·R·T
Where;
P = The pressure of the gas
V = The volume of the gas
R = The universal gas constant
T = The temperature of the gas
n = The number of moles of the gas
Therefore, T = P·V/n·R
By substituting the known values of the variables, we get;
T = 130×1/(3.740648 × 0.08206) ≈ 423.51129 K
The temperature of the gas, T ≈ 423.51129 K
(b) The Van der Waals equation of state;
\(n \cdot R \cdot T = \left (P + a \cdot \dfrac{n^2}{V^2} \right) \cdot (V - n\cdot b)\)
Therefore, we have;
\(T = \dfrac{ \left (P + a \cdot \dfrac{n^2}{V^2} \right) \cdot (V - n\cdot b)}{n \cdot R}\)
Therefore, we have;
\(T = \dfrac{ \left (130 + 2.253 \times \dfrac{3.740648^2}{1^2} \right) \times (1 - 3.740648\cdot 0.04278)}{3.740648 \times 0.08206} = 442.00558 \ K\)
The temperature of the gas, T ≈ 442.00558 K.
As you move down a group of the periodic table how does the atomic radius of the atoms change
Answers
Answer:
An atom gets larger as the number of electronic shells increase; therefore the radius of atoms increases as you go down a certain group in the periodic table of elements. In general, the size of an atom will decrease as you move from left to the right of a certain period.
Explanation:
Isotope problem help me

Answers
Answer:
59 Sc 0
21
tiene numero atomico 21, porque es neutro, y tiene numero masico 59 porque 21 + 28 =59
i need help pls!!!!!!!!!!!
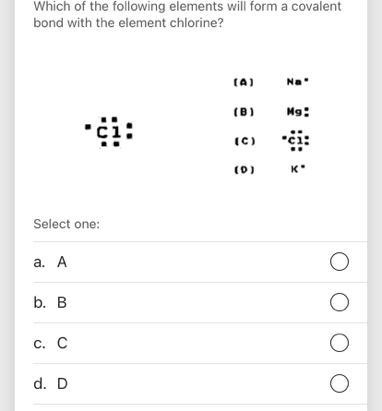
Answers
Which element will form covalent bonds with chlorine?
A. Carbon. YES. C is a nonmetal. B. Magnesium. NO. Mg is a metal. C. Potassium. NO. K is a metal. D. Aluminum. NO. Al is a metal.
Chlorine will form covalent bonds with A. Carbon .

How is the glucose level in the blood maintained? Which body system, organs, and hormones are used and what do they do?
Answers
Answer:
Explanation:
Insulin is an hormone used to regulate blood glucose, as it helps to maintain a balance. It allows for transport of glucose to organs such as liver.
The process of glucose regulation is a complex process. When food is eating glucose is absorbed into the bloodstream this happen from the gut and it raises the blood glucose level this causes insulin(hormone) to be released from the pancreas so glucose can move inside the cells and be used.
As glucose moves inside the cells, the glucose level inside the bloodstream returns to normal and insulin release slows down.
Glucose which is the main energy source used by cells is allowed to be taken up by muscles, liver and fat (adipose tissue) and use as a source of energy so they can function properly.
Help please List the methods you think is used to treat water for contaminants before it gets to your
faucet.
Answers
But if your drinking what out a faucet just boil the what it should do the trick
What is the primary type of heat transfer?: A cool breeze blows off the water.
Answers
Answer:
Explanation:
Heat transfer occurs by three main mechanisms: conduction, where rigorously vibrating molecules transfer their energy to other molecules with lower energy; convection, in which the bulk movement of a fluid causes currents and eddies that promote mixing and the distribution of thermal energy
Which statement best describes what happens to the carbon dioxide produced by humans?.
Answers
Which of the following sequences of elements would all have four valence electrons?
A.) B, Si, As, Te
B.) C, Si, Ge, Sn
C.) O, S, Se, Te
D.) Ga, Ge, As, Se
Answers
Answer:
Your answer should be B.) C, Si, Ge, Sn! I hope this helps you! :)
Write the empirical formula of at least four binary ionic compounds that could be formed from the following ions:
Ca2+,V5+,Cl-,S2-
Answers
The empirical formulas for at least four binary ionic compounds that can be formed from the given ions are as follows: CaF2, CaS, PbF4, and PbS2.
A binary ionic compound is made up of ions from two different elements: one metal and one nonmetal. Iron(III) iodide, FeI3, for example, is made up of iron ions, Fe3+ (elemental iron is a metal), but also iodide ions, I- (elemental iodine is a nonmetal).
The positive ion (cation) has been written first in the name, followed by the negative ion (anion). The cation has the same name as the (neutral metal) element that it is derived. The charge on the cation is denoted by a Roman numeral within parentheses immediately following the cation's name (e.g., Fe3+ = "iron(III)", Fe2+ = "iron(II)"). The anion is named by prefixing the root of (nonmetal) element name with the suffix -ide
To know more about the Ionic compounds, here
https://brainly.com/question/29005103
#SPJ4
Identify how many significant figures each number has. a. 297 b. 549 c. 4678 d. 327 e. 3006 f. 902 g. 8020 h. 0.0000053 1. 0.00002 j. 0.000506 k. 4000 1. 4000.0 m. 7.12 n. 3.05
Answers
Explanation:
a. 3 significant figures
b. 3
c. 4
d. 3
e. 4
f. 3
g. 3
h. 2
i. 1
j. 3
k. 1
l. 6
m. 3
n. 3