true or false even though the full oxidation of glucose is exergonic, some of the reactions in glycolysis are endergonic.
Answers
The statement "Even though the full oxidation of glucose is exergonic, some of the reactions in glycolysis are endergonic" is a TRUE statement.
Glycolysis is the metabolic pathway of reactions that convert glucose into pyruvate (in the presence of oxygen) or lactate (in the absence of oxygen). There are ten sequences of reactions in it catalyzed by enzymes.
The overall reactions of glycolysis are exergonic, which means energy is released into its surroundings, However, some of the reactions within glycolysis absorb energy instead of releasing energy, making them endergonic reactions. One of them is the second reaction in glycolysis, where isomerase catalyzes the isomerization of G-6-P to F-6-P.
Learn more about glycolysis at https://brainly.com/question/737320
#SPJ4
Related Questions
Zn + 2HCl → ZnCl₂ + H₂
How many moles of hydrogen are
produced from the reaction of three
moles of zinc with an excess of
hydrochloric acid?
STUDENT
Answers
Answer:
The balanced chemical equation you provided is:
Zn + 2HCl → ZnCl₂ + H₂
From the equation, we can see that for every 1 mole of zinc (Zn) reacting, 1 mole of hydrogen gas (H₂) is produced. Therefore, the mole ratio of zinc to hydrogen is 1:1.
If you have 3 moles of zinc, you will produce an equal number of moles of hydrogen gas. Therefore, when 3 moles of zinc react with an excess of hydrochloric acid, 3 moles of hydrogen gas will be produced.
Explanation:
Based on their electrons dot diagrams, what is the formula for the covalently bonded compound
of nitrogen and hydrogen?
Answers
Answer:
Nitrogen, the next nonmetal, has 5 electrons in the valence shell, so it needs to combine with 3 hydrogen atoms to fulfill the octet rule and form a stable compound called ammonia (NH3).

The covalent compound formed from nitrogen and hydrogen is called ammonia with the formula NH₃. Each hydrogen shares its valence electron with three valence electrons of nitrogen.
What is ammonia?Ammonia, NH₃ is a covalent compound formed by the combination of nitrogen and three hydrogen atoms. Nitrogen is a highly electronegative element with 5 valence electrons.
Nitrogen needs 3 more electrons to achieve octet. Hence valency of nitrogen is 3. During chemical bonding it gains three electrons though sharing or donation from metals.
Hydrogen needs one more electron to be stable. Hence, nitrogen shares its three valence electrons each with three hydrogens and each hydrogen in turn shares its one valence electron with nitrogen forming NH₃.
Find more on ammonia:
https://brainly.com/question/15409518
#SPJ2
In what ways does science benefit society.
Answers
Answer:It has a specific role, as well as a variety of functions for the benefit of our society: creating new knowledge, improving education, and increasing the quality of our lives. Science must respond to societal needs and global challenges.
Explanation:Science is valued by society because the application of scientific knowledge helps to satisfy many basic human needs and improve living standards. Finding a cure for cancer and a clean form of energy are just two topical examples. Education could become the most important application of science in the next decades.
Which planet could support human life

Answers
Answer:
D
Explanation:
It states that D has oxygen, and is closer to the Sun.
Hope this helped, and please mark as Brianliest <3
Answer:
I am pretty sure it is B
Explanation:
The others would either burn u like a crispy chicken nugget or freeze you like a popsicle
Which molecule has the shortest carbon-oxygen bond length?
A. CH3COOH
B. CH3CH2OH
C. CO₂
D. CO
Answers
What does it mean if a solution is a "weak acid "? Strong acid? Give logical ph values for each
Answers
Answer:
A weak acid is an acid that partially dissociates into its ions in an aqueous solution or water. In contrast, a strong acid fully dissociates into its ions in water. At the same concentration, weak acids have a higher pH value than strong acids.
Explanation:
:)
Branched-chain amino acids (bcaas), commonly known as a supplement to support recovery and stimulate muscle growth, may also serve to:_____.
Answers
BCAA supplements are commonly taken to boost muscle growth and enhance exercise performance.
They may also help with weight loss and reducing fatigue after exercise. This article contains all the most important information about branched-chain amino acids and their benefits.
What are purposes of Branched-chain amino acids (bcaas) ?Leucine, isoleucine, and valine are the three essential amino acids that make up the branched-chain amino acids (BCAAs).Since they are vital, your body cannot create them on its own and you must get them through food.BCAA supplements have been demonstrated to increase muscular mass, lessen muscle pain, and reduce muscle fatigue.They have also been used successfully in hospitals to lessen liver disease symptoms and stop or slow down muscle loss.However, since the majority of people consume enough BCAAs from their diets, taking supplements is unlikely to provide any further advantages.To view more about amino acids, refer to:
https://brainly.com/question/15969022
#SPJ4
A pipe 10 m long and of radius r = 7 cm is to be coated by insulation material to a thickness of dr = 2 mm. Approximate the volume V of insulation material required in m³. Please use pi for л (rather than a decimal approximation) in your answer. Insulation volume (m³): You have not attempted this yet
Answers
The volume of insulation material required is approximately 0.003606 cubic meters (m³).
To calculate the volume of insulation material, we can subtract the volume of the inner pipe (original pipe) from the volume of the outer pipe (original pipe + insulation).
Given:
Length of the pipe, L = 10 m
Radius of the pipe, r = 7 cm = 0.07 m
Thickness of the insulation, dr = 2 mm = 0.002 m
The outer radius of the larger pipe is R = r + dr.
Using the formula for the volume of a cylinder, V = π(R² - r²)L, we can substitute the values and calculate:
V = π((0.07 + 0.002)² - 0.07²) × 10
V ≈ 3.606 × 10⁻³ m³
Therefore, the volume of insulation material required is approximately 0.003606 m³ (cubic meters).
Learn more about cylinder volumes from the given link
https://brainly.com/question/28058531
#SPJ11.
Add coefficients to the reaction summary to show the net results of glycolysis. glucose +aADP+bPi+cNAD+⟶x pyruvate +yATP+zNADH You do not need to add the water and hydrogen ions necessary to balance the overall reaction. a= b= c= x= y= z= Draw the structure of pyruvate at pH7.4.
Answers
At pH 7.4, pyruvate exists in its anionic form, known as pyruvate anion or pyruvate ion structure is (CH3COCOO-).
The net reaction of glycolysis, including coefficients, can be summarized as follows:
Glucose + 2 ADP + 2 Pi + 2 NAD+ ⟶ 2 Pyruvate + 2 ATP + 2 NADH
Here are the values for the coefficients:
a = 2 (since 2 ADP molecules are consumed)
b = 2 (since 2 Pi molecules are consumed)
c = 2 (since 2 NAD+ molecules are consumed)
x = 2 (since 2 pyruvate molecules are produced)
y = 2 (since 2 ATP molecules are produced)
z = 2 (since 2 NADH molecules are produced)
To draw the structure of pyruvate at pH 7.4.
Pyruvate is a three-carbon molecule with the chemical formula C3H4O3.
At pH 7.4, pyruvate exists in its anionic form, known as pyruvate anion or pyruvate ion (CH3COCOO-).
Here is a simplified structural representation of pyruvate at pH 7.4:
In the structure, the carbon skeleton consists of three carbon atoms, with a carbonyl group (C=O) attached to one carbon and a carboxylate group (-COO-) attached to another carbon.
The remaining carbon is bonded to a hydrogen atom.
The negative charge (represented by the "-") is present on the oxygen atom, indicating the anionic form of pyruvate.
Learn more about pyruvate from the given link:
https://brainly.com/question/16346028
#SPJ11

Nickel crystallizes in a face-centered cubic structure, its density is 8.9 g cm−3. Calculate the radius (in A˚) of the nickel atom. [Given that the atomic weight of Ni is 58.89 amu.]A. 2.4B. 3.2C. 1.2D. 0.8
Answers
Nickel crystallizes in a face-centered cubic structure, its density is 8.9 g cm−3. The radius (in 1.2A˚) Option C is Correct.
The formula for calculating the radius (r) of an atom in a face-centered cubic structure is:
\(r=\frac{a}{2} \sqrt{2}\)
Where "a" is the edge length of the unit cell. The density of nickel is given as 8.9 g/cm³, which can be converted to g/m³ by multiplying by 1000:
8.9 g/cm³ = 8900 g/m³
The atomic weight of nickel is given as 58.89 amu. This means that the mass of one nickel atom is:
58.89 g/mol / 6.022 x 10²³ atoms/mol = 9.77 x 10⁻²³ g/atom
Now we can use the equation:
density = (mass of unit cell) / (volume of unit cell)
The unit cell of a face-centered cubic structure contains 4 atoms, so the mass of the unit cell is:
mass of unit cell = 4 x 9.77 x 10⁻²³ g/atom = 3.908 x 10⁻²² g
The volume of the unit cell can be calculated as:
volume of unit cell = (a/2)³
Substituting the values and solving for "a":
8900 g/m³ = 3.908 x 10⁻²² g / ((a/2)³)
a = 0.352 nm
Finally, we can calculate the radius of the nickel atom using:
r = (a/2) ×√(2)
r = (0.352/2) × √2) = 0.124 nm = 1.24 A˚
Therefore, the answer is (C) 1.2.
Learn more about unit cell here
https://brainly.com/question/30065994
#SPJ11
1. Patient is admitted with neck injury which has resulted in a loss of function in their thyroid.
Predict how this damage will affect hormone levels (Select all that apply)
A. Thyroid hormone levels will increase
B. Thyroid hormone levels with decrease
C TSH levels will increase
DTSH levels will decrease
Answers
Answer:
A. (i think)
Explanation:
3
Li
6.941
Atomic # =
Atomic Mass =
# of Protons =
# of Neutrons =
# of Electrons =
Answers
Answer:
36.94133.23Explanation:
pls mark brainliest
Consider the following B+-decay: p < n + et + ve Question 2. What is the name of the interaction which is involved in the B+-decay? Question 3. What are the conserved quantities in the reaction above? Is the quark flavour a conserved quantity?
Answers
2. The interaction involved in the B⁺-decay is known as beta decay.
3. The conserved quantities in the reaction are:
Conservation of electric chargeConservation of lepton numberConservation of baryon numberThe quark flavor is not a conserved quantity in the given reaction of B⁺-decay.
The B⁺-decay is a type of beta decay, specifically beta plus decay. In beta plus decay, a proton (p) decays into a neutron (n), emitting a positron (e+) and an electron neutrino (νe):
p → n + e⁺ + νe
2. The interaction involved in the B⁺-decay is the weak nuclear force. The weak force is responsible for processes involving the transformation of particles, such as the conversion of a proton into a neutron in this case.
The interaction involved in the B⁺-decay is known as beta decay. Specifically, the B⁺-decay refers to the decay of a positively charged (B⁺) meson, which is a type of subatomic particle.
3. The conserved quantities in the reaction are:
Conservation of electric charge: The total charge on both sides of the reaction is conserved. The proton (p) has a charge of +1, while the neutron (n) has no charge. The positron (e⁺) has a charge of +1, which balances out the charge.
Conservation of lepton number: The total lepton number is conserved in the reaction. The lepton number of the proton and neutron is 0, while the lepton number of the positron and electron neutrino is also 0. Hence, the lepton number is conserved.
Conservation of baryon number: The baryon number is conserved in the reaction. The baryon number of the proton is 1, and the baryon number of the neutron is also 1. Therefore, the total baryon number is conserved.
Regarding quark flavor, it is not conserved in the B⁺-decay. The decay process involves the transformation of a up-type quark (u) in the proton to a down-type quark (d) in the neutron. This change in quark flavor is allowed by the weak force.
Learn more about Weak Nuclear Force at
brainly.com/question/31753095
#SPJ4
A heat sensing resistor that changes its value as its temperature changes is known as a:_____.
Answers
A heat sensing resistor that changes its value as its temperature changes is known as a Thermistor.
The Thermistor is a special type of variable resistive detail that adjustments its physical resistance while uncovered to modifications in temperature. The Thermistor is a solid kingdom temperature sensing device which acts a chunk like an electrical resistor however is temperature touchy. A thermistor is a temperature sensitive resistor. they may be regularly used as a temperature sensor.
Thermocouples are the maximum generally used form of temperature sensor. they are used in business, automotive, and purchaser programs. Thermocouples are self-powered, require no excitation, can function over a extensive temperature variety, and feature brief response times.
Learn more about Thermistor here:-https://brainly.com/question/14531016
#SPJ4
what happens to the speed of a sound wave from an underwater animal as the sound passes into the air above?
A. It stays the same
B. It falls to zero
C. It decreases
D. It increases

Answers
i believe so.
a scientist begins with 375 grams of a radioactive substance. after 200 minutes, the sample has decayed to 38 grams. to the nearest hundredth of a minute, what is the half-life of this substance?
Answers
The half-life of the substance is approximately 62.43 minutes.
To calculate the half-life of the substance, we need to use the formula:
N = N₀(1/2)^(t/T)
where N is the remaining amount of the substance, N₀ is the initial amount of the substance, t is the time elapsed, and T is the half-life.
Plugging in the given values,
38 = 375(1/2)^(200/T)
T ≈ 62.43 minutes
Therefore, the half-life of the substance is approximately 62.43 minutes. This means that it takes 62.43 minutes for half of the substance to decay.
To know more about the half-life, here
brainly.com/question/14250693
#SPJ4
What volume (in milliliters) of 0. 130 M NaOH should be added to a 0. 120 L solution of 0. 021 M glycine hydrochloride (pKa1 = 2. 350, pKa2 = 9. 778) to adjust the pH to 2. 63?What volume (in milliliters) of 0. 130 M NaOH should be added to a 0. 120 L solution of 0. 021 M glycine hydrochloride (pKa1 2. 350, pKa 9. 778) to adjust the pH to 2. 63?NaOH volume =____ mL
Answers
The volume of 0.130 M NaOH required to adjust the pH of the solution to 2.63 is 15.4 mL.
pH = pKa + log([A-]/[HA])
[H+] = \(10^{-pH}\) = \(10^{-2.63}\) = 1.33 x \(10^-3\)M
Using the equation for the dissociation constant of a weak acid, we can calculate the concentration of A-:
Ka = [H+][A-]/[HA]
Ka = \(10^{-pKa1}\) = 1.67 x \(10^-3\)
[A-]/[HA] = Ka/[H+] = 1.67 x \(10^-3\) / 1.33 x \(10^-3\) = 1.256
[A-] = [HA] x 1.256 = 0.026 M
Now we can use the Henderson-Hasselbalch equation to calculate the required volume of NaOH to adjust the pH to 2.63. At pH 2.63, the ratio of [A-]/[HA] should be equal to \(10^(pH-pKa1)\)= 1.63 x 10³:
1.63 x 10³ = [A-]/[HA] = ([0.026 + x]/0.12)/(0.021)
where x is the amount of NaOH (in moles) added to the solution. Solving for x, we get:
x = 0.12 * 1.63 x 10³ * 0.021 - 0.026 = 0.002 M
To convert moles of NaOH to milliliters of a 0.130 M solution, we can use the following equation:
moles NaOH = Molarity x volume (in liters)
0.002 M = 0.130 M x (volume / 1000)
volume = 15.4 mL
pH is a measure of the acidity or basicity of a solution. It is defined as the negative logarithm (base 10) of the concentration of hydrogen ions (H+) in a solution. The pH scale ranges from 0 to 14, with a pH of 7 being considered neutral. A pH below 7 indicates acidity, with lower numbers indicating greater acidity, while a pH above 7 indicates alkalinity, with higher numbers indicating greater alkalinity.
Acids are substances that donate H+ ions, increasing the concentration of H+ in a solution and lowering its pH. Bases, on the other hand, are substances that accept H+ ions, decreasing the concentration of H+ and raising the pH. The pH of a solution can have a significant impact on chemical reactions and biological processes, as many enzymes and other biomolecules are sensitive to changes in pH. Therefore, maintaining the appropriate pH is crucial in many chemical and biological applications.
To learn more about pH visit here:
brainly.com/question/491373
#SPJ4
Use this graphic to explain how matter is conserved in a nuclear reaction

Answers
Nuclear processes alter the sorts of atoms present, but chemical reactions do not. The electrons in the atom play a significant role in chemical reactions when nucleus reactions occur in atom nuclei.
What is nuclear explain?The energy found in an atom's nucleus, or core, is known as nuclear energy. Energy maintains the nucleus of atoms together, the minuscule units that make up all matter in the universe. The dense nucleus of an atom has an enormous quantity of energy.
Why is a nuclear threat a threat?The most lethal weapons on earth are nuclear weapons. One can wipe out an entire metropolis, perhaps killing millions of people, endangering the ecosystem and the lives of future generations due to its long-term devastating impacts.
To know more about Nuclear visit:
https://brainly.com/question/18187269
#SPJ1
lysine is a compound composed of carbon, hyrgoen, nitgrogen, and oxygen. when 1.50 g of lysine i burned, 2.72 g of carbon dioxide, 1.29 g of water, and 0.287 g of nitrogen gas are produced. what is the empirical formula of lysine? if the molar mass of lysine is 146.19 g/mol. what is the molecular formula?
Answers
The empirical formula of lysine is C₆H₁₄N₂.
By calculating the smallest whole number ratio from the mass percentages of a compound's constituent parts, it is possible to calculate its empirical formula.
We must first establish how many moles of each element are included in 1.50 g of lysine in order to determine the empirical formula for lysine. The mole ratios of the elements may then be calculated using these values and will match the ratio of the subscripts in the empirical formula.
We may determine how many moles of carbon and hydrogen are contained in 1.50 g of lysine by computing the mass of generated CO₂ and H₂O.
Moles of C = 2.72 g CO₂ x (1 mol CO₂ / 44.01 g CO₂) = 0.062 moles
Moles of H = 1.29 g H₂O x (2 moles H / 18.02 g H₂O) = 0.143 moles
Secondly, by being aware that lysine also generated 0.287 g of N. We can determine how many moles of N there are.
moles of N = 0.287 g N x (1 mole N / 28.02 g N) = 0.0102 moles
By dividing each element by the minimum number of moles, which is 0.0102 moles, we can now determine the ratio of the elements.
C = 0.062 moles / 0.0102 moles = 6
H = 0.12 moles / 0.0102 moles = 14
N = 0.0102 moles / 0.0102 moles = 1
Therefore, the empirical formula of lysine is C₆H₁₄N₂
Learn more about empirical formula at https://brainly.com/question/14044066
#SPJ4
Which gas would be least Ideal at the same conditions of
temperature and pressure?
a He(9)
b CO(g)
c O2(9)
d H20(9)
Answers
Perceived control over a stressful event results in Group of answer choices a. less reported stress b. more frustration regarding the stressful event c. less motivation to solve the problem causing the stress d. increased arousal
Answers
Perceived control over a stressful event typically results in a. less reported stress.
When an individual believes they have control over a situation, they tend to feel more empowered and confident in their ability to manage or resolve the stressor. This perception of control allows them to develop effective coping strategies and focus on problem-solving rather than being overwhelmed by the stress. Consequently, this sense of control often leads to a reduction in overall stress levels.
Contrary to options b, c, and d, perceived control does not typically lead to more frustration, less motivation to solve the problem, or increased arousal. Instead, having a sense of control often allows individuals to approach the stressful event in a more positive and proactive manner, ultimately reducing the negative impact of the stressor on their mental and emotional well-being. Thus, fostering a sense of control over stressful events can be a crucial factor in managing stress effectively. So therefore the correct answer is a. less reported stress.
Learn more about stress at
https://brainly.com/question/31366817
#SPJ11
You mix 2 moles of hbr with 3 moles of koh in enough water to make 1 l of solution. how much kbr do you expect to make?
Answers
If we mixed 3 moles of HBr with 2 moles of KOH in enough water to make 1 liter of solution, then the amount of KBr that would be formed would be 3 moles.
The balanced chemical equation for the reaction between HBr and KOH is:
HBr + KOH → KBr + H2O
In the given case, we have 3 moles of HBr and 2 moles of KOH
Their mole ratio = 1.5:1.
This means that for every 1.5 moles of HBr, we have 1 mole of KOH, which will be enough to react with all the HBr.
So, the amount of KBr formed would be 3 moles, which is the same as the amount of HBr that was added to the reaction.
To learn more about potassium hydroxide (KOH):
https://brainly.com/question/28330489
#SPJ4
24. 00 ml of a 0. 25 m naoh solution is titrated with 0. 10m hcl. What is the ph of the solution after 24. 00 ml of the hcl has been added?.
Answers
pH of the solution after 24. 00 ml of the hcl has been added is 12.87
millimoles NaOH = mL x M = 24.00 mL x 0.25 M = 6.00
millimoles HCl = 24.00 mL x 0.10 M = 2.40
total volume = 48.00 mL
.................................NaOH + HCl ==>NaCl + H2O
initial.........................6.00.........0............0.........0
added.....................................2.40............................
change.................... -2.40......-2.40.........+2.40.... +2.40
equilibrium.................3.60.........0..............2.40.......2.40
The NaCl contributes nothing to the pH of the final solution. The pH is determined by the excess of NaOH present. (NaOH) = millimoles/mL = 3.60/48.00 = 0.075 M = (OH^-)
pOH = -log (OH^-). Then
pOH = -log (0.075)
pOH =1.1249
As we know,
pH + pOH = pKw = 14.00
pH=14-pOH
pH=14-1.1249
pH=12.87
What is pH?pH is a logarithmic measure of an aqueous solution's hydrogen ion concentration. pH = -log[H+], where log is the base 10 logarithm and [H+] is the concentration of hydrogen ions in moles per liter.
The pH of an aqueous solution describes how acidic or basic it is, with a pH less than 7 being acidic and a pH greater than 7 being basic. A pH of 7 is regarded as neutral (e.g., pure water). pH values typically range from 0 to 14, though very strong acids may have a negative pH and very strong bases may have a pH greater than 14.
Learn more about pH:
https://brainly.com/question/491373
#SPJ4
Categorize bond types using electronegativity difference.
Answers
The types of bonds that we have are; ionic bond, covalent bond and polar covalent bond.
What is electronegativity difference?The term electronegativity difference has to do with the difference in the ability of the atoms of a bond to attract the electrons of the bond towards itself. There are some compounds that have two atoms in a bond, in which one of the atoms in the bond have the ability to attract the electrons of the bond closer to itself.
The bond types that we have depends on the difference in electronegativity and they are;
Ionic bond; A bond is said to be ionic if the electronegativity difference is above 2.5
Polar covalent; A bond is polar covalent is the electronegativity difference in the bond is above 0.5
Covalent bond: A bond is said to be covalent if the electronegatvity difference of the bond is below 0.5.
Learn more about electronegativity:https://brainly.com/question/17762711
#SPJ1
what is the cathode and anode of k2so4
Answers
O 2
Here,
Electrolysis of H
2
O takes place:
2H
2
O→O
2
+4H
⊕
+4e
−
when water unfreezes what happens to the volume of the sample
it increases
it decreases
it stays the same
Answers
Answer:
It eventually decreases.Explanation:
Frozen water is known to have more volume, but less density. When water slowly gets unfrozen, it'd volume decreases and its density increases. Hence, when the water gets unfrozen, it's volume decreases.
1- Formulas of the reactants: NaOH Cag, Clear solution) Fecha mdecular Equation: fog, light yella Complete Ionie Equation: Net Ionic Equation: Formulas of the possible products: Observation (visual): clear solution - rusty red Per Evidence of Reaction (proof): clear solution - rusty red PPT Spectator Ions? Reacting tons? Did the reaction accur? Yes Classification of Reaction? 2 - Formulas of the reactants: Coci, (aq, pink solution) AgNO, (aq, clear solution) CHEL 111-Metathesis Reaction Revised - S. Hektary & Alka-8/2020
Answers
During the reaction between NaOH and CaCl2, a clear solution of NaOH reacts with CaCl2 to form a rusty red precipitate of Ca(OH)2.
Here is a more detailed answer:
1. Formulas of the reactants:
- NaOH: Sodium hydroxide
- CaCl2: Calcium chloride
2. Complete Molecular Equation:
NaOH(aq) + CaCl2(aq) → Ca(OH)2(s) + 2NaCl(aq)
3. Complete Ionic Equation:
Na⁺(aq) + OH⁻(aq) + Ca²⁺(aq) + 2Cl⁻(aq) → Ca(OH)2(s) + 2Na⁺(aq) + 2Cl⁻(aq)
4. Net Ionic Equation:
Ca²⁺(aq) + 2OH⁻(aq) → Ca(OH)2(s)
5. Formulas of the possible products:
Ca(OH)2: Calcium hydroxide
6. Observation (visual):
Before the reaction, the solution is clear. After the reaction, a rusty red precipitate is formed.
7. Evidence of Reaction (proof):
The change in color from a clear solution to a rusty red precipitate indicates that a chemical reaction has occurred.
8. Spectator Ions:
Na⁺(aq) and Cl⁻(aq) are spectator ions because they do not participate in the overall reaction. They are present on both sides of the equation.
9. Reacting Ions:
Ca²⁺(aq) and OH⁻(aq) are the reacting ions because they undergo a chemical change to form the precipitate.
10. Did the reaction occur?
Yes, the reaction occurred. The formation of a precipitate indicates a chemical reaction has taken place.
11. Classification of Reaction:
This is a double displacement or metathesis reaction, where the ions of the reactants exchange to form new compounds. In this case, calcium hydroxide (Ca(OH)2) precipitates out of the solution.
To know more about precipitate, refer here:
https://brainly.com/question/30904755
#SPJ4
In the heating and cooling curves below, identify the letter in the diagram diagram that corresponds to each of the listed processes in the table
I’m so confused if anyone could help (and explain as if I’m a 3 yr old) that would be helpful
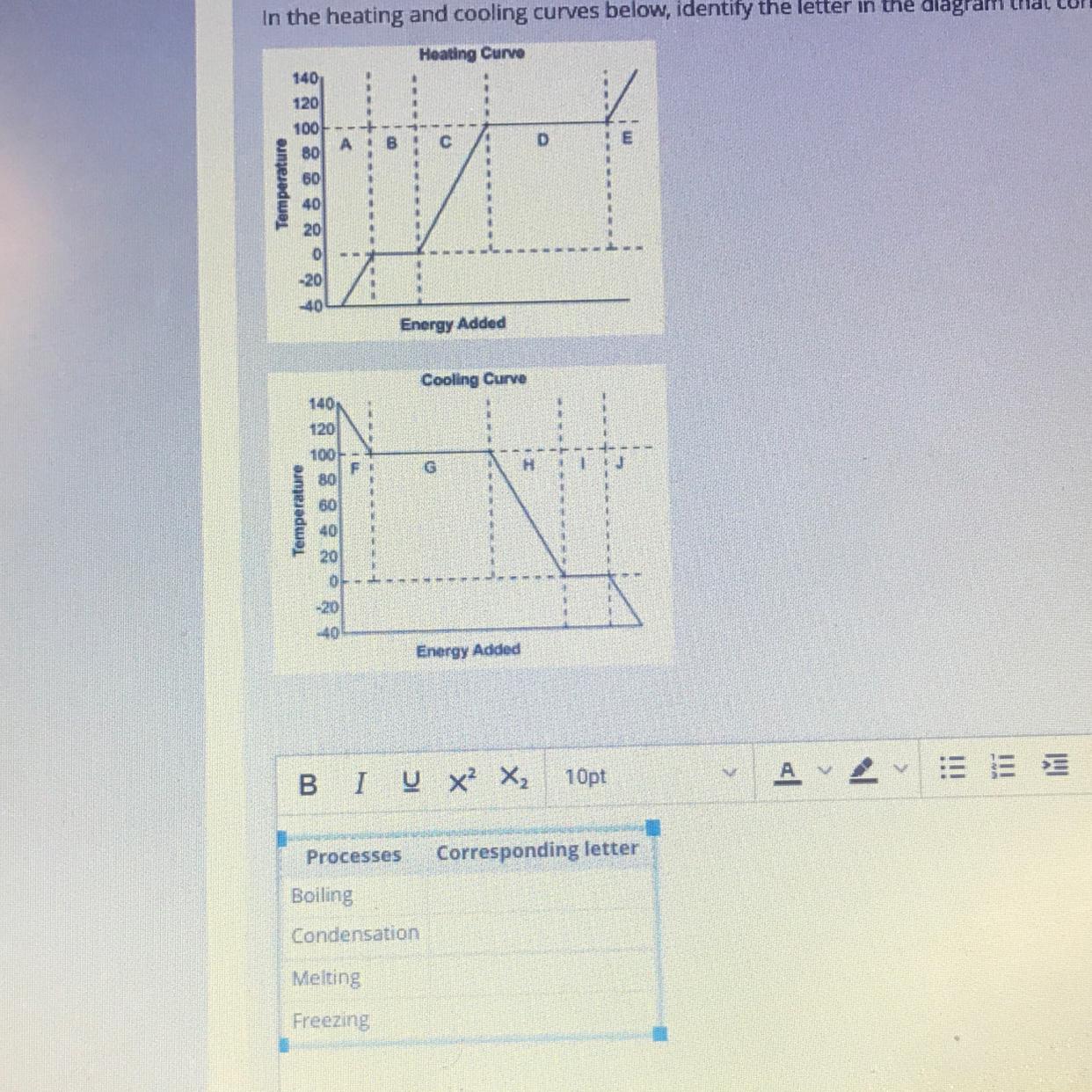
Answers
Answer:
Test for the first one is the best for
There are three stable forms of neon: neon-20, neon-21, and neon-22. Which statement is true?
Answers
Answer:
three stable isotopes
Neon has three stable isotopes: 20Ne (90.48%), 21Ne (0.27%) and 22Ne (9.25%). Ne and 22Ne are partly primordial and partly nucleogenic (i.e. made by nuclear reactions of other nuclides with neutrons or other particles in the environment) and their variations in natural abundance are well understood.
The statement that is true is the charge of all isotopes are same. The correct option is D.
What are isotopes?Isotopes are members of an element family that have the same number of protons but differ in terms of neutrons.
The atomic number of an element on the Periodic Table is determined by the number of protons in its nucleus.
There are three isotopes of neon. Since there is no significant global production of these isotopes, the more plentiful 20Ne and 22Ne are essentially all primordial.
Isotopes can form naturally through radioactive decay of a nucleus or unnaturally by bombarding a stable nucleus with charged particles in a nuclear reactor using accelerators or neutrons.
Thus, the correct option is D as they all have same charge.
For more details regarding isotopes, visit:
https://brainly.com/question/11680817
#SPJ2
Your question seems incomplete, the missing options are:
- the atomic mass of the three isotopes is the same
-the three isotopes are all radioactive
-the three isotopes are equally abundant in nature
-the charge of all isotopes is the same
The phase that occurs beyond the critical point is:
A. Liquid
B. Solid
C. Gas
D. All of the above
Answers
Answer:
C. Gas
Explanation:
A supercritical fluid occurs beyond the temperature of critical point, wherein the state matter transitions from liquid to gaseous phase interchangeably. Whereas, triple point occurs when all three states of matter: solid, liquid nd gas; coexist.
Hence the answer to the question is Gaseous phase/
I hope you found this helpful.