To what volume should you dilute 133 mL of an 8.00 M CuCl2 solution so that 51.0 mL of the diluted solution contains 4.24 g CuCl2
Answers
To dilute 133 mL of an 8.00 M CuCl₂ solution to obtain a concentration of 4.24 g CuCl₂ in 51.0 mL of the diluted solution, the solution should be diluted to a volume of approximately 203 mL.
To calculate the volume of the diluted solution, we can use the concept of the dilution equation, which states that the initial moles of solute (CuCl₂) are equal to the final moles of solute in the diluted solution.
Given that the concentration of the initial solution is 8.00 M and the volume is 133 mL, we can determine the initial moles of CuCl₂ by multiplying the concentration by the volume: 8.00 mol/L x 0.133 L = 1.064 mol.
Next, we can use the final concentration (4.24 g/51.0 mL) to determine the final moles of CuCl₂ in the diluted solution. First, convert the mass of CuCl₂ to moles using its molar mass: 4.24 g / 134.45 g/mol = 0.0316 mol.
Since the initial and final moles of CuCl₂ are equal, we can set up the equation: 1.064 mol = 0.0316 mol/Vfinal.
Rearranging the equation, we find that Vfinal = 1.064 mol / 0.0316 mol/mL = 33.8 mL.
However, the question asks for the total volume of the diluted solution, so we add the volume of the initial solution to the calculated volume: 33.8 mL + 133 mL = 166.8 mL.
Therefore, the solution should be diluted to a volume of approximately 203 mL (166.8 mL + 51.0 mL) to achieve the desired concentration of 4.24 g CuCl₂ in 51.0 mL of the diluted solution.
To learn more about diluted solution, here
https://brainly.com/question/15467084
#SPJ4
Related Questions
the volume of an unknown liquid is measured to be 25 ml. the mass of the liquid is 20. grams. what is the density of the liquid?
Answers
The density of the liquid is 0.8 g/ml, that can be calculated by substituting the values in density formula.
Density can be defined as the ratio of mass over volume. It can be expressed as follows:
Density= Mass/Volume
There can be various units to define density but the standard unit of density is g/ml.
The volume of the liquid = 25 ml
The mass of the liquid =20 grams
Density= 20/25
Density=0.8 g/ml
Therefore, the density of the liquid is 0.8 g/ml.
To learn more about density check the link below:
https://brainly.com/question/1354972
#SPJ4
Combustion analysis of an unknown compound containing only carbon and hydrogen produced 2.845 g of co2 and 1.744 g of h2o. what is the empirical formula of the compound?
Answers
The empirical formula of the unknown compound is CH₂O.
To determine the empirical formula of the unknown compound, we need to find the mole ratios of carbon and hydrogen in the compound based on the given masses of carbon dioxide (CO₂) and water (H₂O) produced during combustion analysis.
First, we calculate the moles of CO₂ and H₂O produced:
Moles of CO₂ = mass of CO₂ / molar mass of CO₂
= 2.845 g / 44.01 g/mol
= 0.0647 mol
Moles of H₂O = mass of H₂O / molar mass of H₂O
= 1.744 g / 18.015 g/mol
= 0.0968 mol
Next, we determine the mole ratio of carbon to hydrogen by dividing the moles of each element by their respective smallest values:
Carbon: 0.0647 mol / 0.0647 mol = 1
Hydrogen: 0.0968 mol / 0.0647 mol = 1.5
We need to simplify the ratio, so we multiply both values by 2 to obtain whole numbers:
Carbon: 1 × 2 = 2
Hydrogen: 1.5 × 2 = 3
The empirical formula of the unknown compound is CH₂O, indicating that it contains 2 carbon atoms, 3 hydrogen atoms, and 1 oxygen atom.
In summary, the empirical formula of the unknown compound is CH₂O.
For more such questions on empirical formula
https://brainly.com/question/1603500
#SPJ4
What is the ability for a substance to flow
Answers
The ability for a substance to flow is fluidity.
Which substances show flowing?Only liquid and gaseous substances have the ability to flow from one point to other as they have weak intermolecular force in it.
Fluidity is the quantity which gives idea about the ability of that substance to flow. At normal conditions at room temperature, any substance has the tendency to distort its shape in order to flow or take the shape of the container it is enclosed in.
Hence the ability for a substance to flow is fluidity.
To know more about fluidity, visit the below link:
https://brainly.in/question/466917
#SPJ2
4
Isotopes are atoms of the same element with the same number of
protons and
(0.5 Points)
O a different number of neutrons.
a different number of electrons
a different number of molecules
the same number of neutrons
Answers
Answer:
a. is tae answer
Explanation:
tamapoyang sagot ko)
If you had a 4.0M and a 0.4M solution of nitric acid, which would have a lower pH? Why?
Answers
Answer:
the second one would have lower
Explanation:
because of the amount
pH is the measure of the negative log of the hydrogen ion concentration. The 0.4 M nitric acid will have lower pH.
What is pH?pH is the concentration of the hydronium or the hydrogen ion in the water that gives the account to the basic and acidic nature of the substance.
pH is dependent on the concentration of the substance as the increased concentration increases the pH while the lower concentration lowers the pH.
Therefore, 0.4 M will have a lower concentration.
Learn more about pH here:
https://brainly.com/question/27036490
#SPJ2
quid water (h2o). rank the quantities of energy input required to produce the following changes from the largest to the smallest. in your ranking, note any cases of equality. (use only the symbols > or
Answers
So I entered the answer, which is a=b=c=e>d, but it says it's incorrect. the final temperature from the initial temperature, multiplying it by the mass, and multiplying it again by the water's specific heat.
Why is water's specific heat so high?
Hydrogen bonds between water molecules are what give water its remarkable thermal conductivity. Hydrogen bonds are disrupted and water molecules can move freely when heat is absorbed. Hydrogen bonds are created and release a significant quantity of energy when the temperature of water drops.
Why does temperature-related specific heat rise?
The average kinetic energy of the molecules increases as the substance heats up. The collisions generate sufficient energy to support rotation. Rotation
To know more about specific heat visit:
brainly.com/question/11297584
#SPJ4
you are asked to prepare 100ml of a 1.5m kbr solution. what mass of kbr do you need? show your calculation in the space provided.
Answers
To prepare 100ml of a 1.5m kbr solution. To find mass of kbr we need
we can use the formula:
mass = molarity x volume x molar mass
where molar mass of KBr is 119.00 g/mol.
So,
mass = 1.5 M x 100 ml x (119.00 g/mol) / 1000 ml/L
mass = 1.785 g
Therefore, 1.785 g of KBr is required to prepare 100 ml of a 1.5 M solution.
Potassium Bromide, sometimes known as KBr, is a salt that is commonly used as an anticonvulsant and sedative.
Other names for potassium bromide include Kalii bromidum, tripotassium tribromide, and bromide salt of potassium.
The odourless potassium bromide salt has a sharp, bitter salty flavour and is available as white crystals, colourless crystals, or white granular solids. Aqueous KBr solutions have a pH of 7.
Learn more about Potassium Bromide here :
brainly.com/question/17154705
#SPJ4
Deuterium is an isotope of hydrogen (H) that has:
A. 1 proton and 1 neutron
B. 1 proton and 2 neutrons
C. 1 proton and O neutrons
D. 2 protons and 1 neutron
Answers
Answer: B. 1 proton and 1 neutron(s)
Explanation: Founders Educere answer.
I love you, fellow summer school students good luck!
Please rate and vote and mark brainliest, I want a rank up.
5H₂
1. What number represents the coefficient?
2. What number represents the subscript?
3. What element is represented by the letter H?
4. How many total H’s are there?
Answers
Answer:
1 H
Explanation:
In chemistry , the chart shows that there is only on Hydrogen ( representing H) therefore the coefficient says two hydrogen and is possible that two hydrogens are used in the formulary for a specific reason
the formation of the aldol condensation product from the initial addition product is favored for all of the following reasons except
Answers
The formation of the aldol condensation product from the initial addition product is favored for all of the following reasons except the condensation product is stable, conjugated alpha, beta-unsaturated ketone
Aldol condensation
Sometimes, either thermally or through acidic or basic catalysis, the adducts produced by the Aldol Addition can be readily transformed (in situ) into,-unsaturated carbonyl compounds.
This spontaneous dehydration is caused by the conjugated system's development. The condensation product can be obtained immediately using a number of procedures without isolating the aldol beforehand.
The Robinson Annulation's second step is the aldol condensation.
Know more about aldol condensation at:
https://brainly.com/question/27178362
#SPJ4
acid-catalysed dehydration of 2,2-dimethylcyclohexanol yields, in part, isopropylidenecyclopentane.
T/F
Answers
The given statement is true that acid-catalysed dehydration of 2,2-dimethylcyclohexanol yields, in part, isopropylidenecyclopentane.
In the acid-catalysed dehydration of 2,2-dimethylcyclohexanol, one of the products formed is isopropylidenecyclopentane. This is because during the reaction, the hydroxyl (-OH) group of the dimethylcyclohexanol molecule is removed, leaving behind a carbocation intermediate.
This intermediate then undergoes a rearrangement to form the isopropylidenecyclopentane product. It is important to note that this is only one of the products that can be formed during this reaction. Other products may include various alkenes, depending on the reaction conditions and the content loaded. Overall, this reaction is an important example of acid-catalysed dehydration, which is a common chemical process used in various industries to produce a wide range of organic compounds.
To know more about dimethylcyclohexanol visit:
https://brainly.com/question/31422384
#SPJ11
how many moles of aluminum metal are required to produce 4.04 l of hydrogen gas at 1.11 atm and 27oc by reaction with hcl?
Answers
The moles of the aluminum metal are required to produce the 4.04 l of hydrogen gas at the 1.11 atm and the 27 °C by reaction with the HCl is 0.060 mol.
The ideal gas equation is as :
P V = n R T
Where,
P = pressure of the gas = 1.11 atm
V = volume of the gas = 4.04 L
T = temperature of the gas = 300 K
R = gas constant = 0.823 L atm K⁻¹ mol⁻¹
n = moles = P V / R T
n = ( 1.11 × 4.04 ) / ( 0.0823 × 300)
n = 0.181 mol of H₂
The chemical equation is :
2Al(s) + 6HCl(aq) ===> 2AlCl₃(aq) + 3H₂(g)
The moles of the Al = (2/6) 0.181 mol
The moles of the Al = 0.060 mol
To learn more about moles here
https://brainly.com/question/31597231
#SPJ4
explain why chemical reactions in the body are often irreversible
Answers
When products of a reaction are continuously taken away from the sight of the reaction it is unable to present for the reverse reaction. Furthermore, reaction involving energy release will not go backwards unless energy is put into them.
Chemical processes that occur within living organisms are referred to as biochemical reactions. A living thing's metabolism is the culmination of all its biochemical processes.
Both catabolic (energy-releasing or exothermic) and anabolic (energy-absorbing or endothermic) events can occur during metabolism.
A biological catalyst termed an enzyme is required for the majority of biochemical reactions to increase their rate. The activation energy required to start the reaction is decreased by enzymes. The majority of proteins that make up enzymes are those that only interact with their substrate, a single kind of material.
Humans can inherit a wide variety of metabolic diseases. A single misplaced or faulty enzyme is the root of the majority of them.
To know more about reverse reaction click here:
https://brainly.com/question/16614705
#SPJ4
What is an example of mass in your house?
Answers
Answer:
any sloid, liquid, or gas in your house.
Explanation:
Molecular geometry datasheet
Please help!! I don’t understand how to do this
Answers
Molecular geometry depends on the shape of molecules present in a compound.
What is molecular geometry?
Molecular geometry can be defined as a three -dimensional arrangement of atoms which constitute the molecule.It includes parameters like bond length,bond angle and torsional angles.
It influences many properties of molecules like reactivity,polarity color,magnetism .The molecular geometry can be determined by various spectroscopic methods and diffraction methods , some of which are infrared,microwave and Raman spectroscopy.
They provide information about geometry by taking into considerations the vibrational and rotational absorbance of a substance.Neutron and electron diffraction techniques provide information about the distance between nuclei and electron density.
Learn more about molecular geometry,here:
https://brainly.com/question/28557524
#SPJ4
under which conditions do you expect helium gas to deviate most from ideal behavior?
Answers
Answer:
Explanation:
[2-0] NC Special Operation
[2] Supervisor
[2-1] Emergency Response
[2-2] Juggernaut
[2-3] Commander
[2-4] Division Director
Energy of a light with a frequency of 3000MHz
Answers
Answer:
A light with a 3000 MHz frequency has an energy of 1.9878 10-24 J.
Explanation:
How are the life cycles of cats and bees alike?
A. The young of both look different from their parents.
B. The young of both look like small adults.
C. Both animals go through metamorphosis.
D. Both follow a pattern of birth, growth, and death.
Answers
Answer:
D
Explanation:
The life cycles of cats and bees alike in the fact that both follow a pattern of birth, growth, and death. Hence, option D is correct.
What is life cycle?The life cycle of an organism is the events of its life starting from its birth to death. Every organisms in earth is passing through the common events that is birth, growth and death.
The events in between these stages may vary from organism to organism. For instance there will be a series of stages in growth for human child which is entirely different from that of a kitten.
The events of growth and its changes in appearance, behavior etc. for a cat is entirely different from that of a bee. Therefore, the common stages for the life cycle of all the organism is the pattern birth, growth and death.
To find more on life cycle of bees, refer here:
https://brainly.com/question/23874222
#SPJ2
pls help asap!! plssss

Answers
AgNO₃+NaCl⇒AgCl+NaNO₃
Further explanationDouble-Replacement reactions. Happens if there is an ion exchange between two ion compounds in the reactant to form two new ion compounds in the product
Reaction
AB + CD⇒AD + CB
So for the option :
1. synthesis/combination reaction
2. decomposition reaction
3. double replacement reaction
4. single replacement reaction
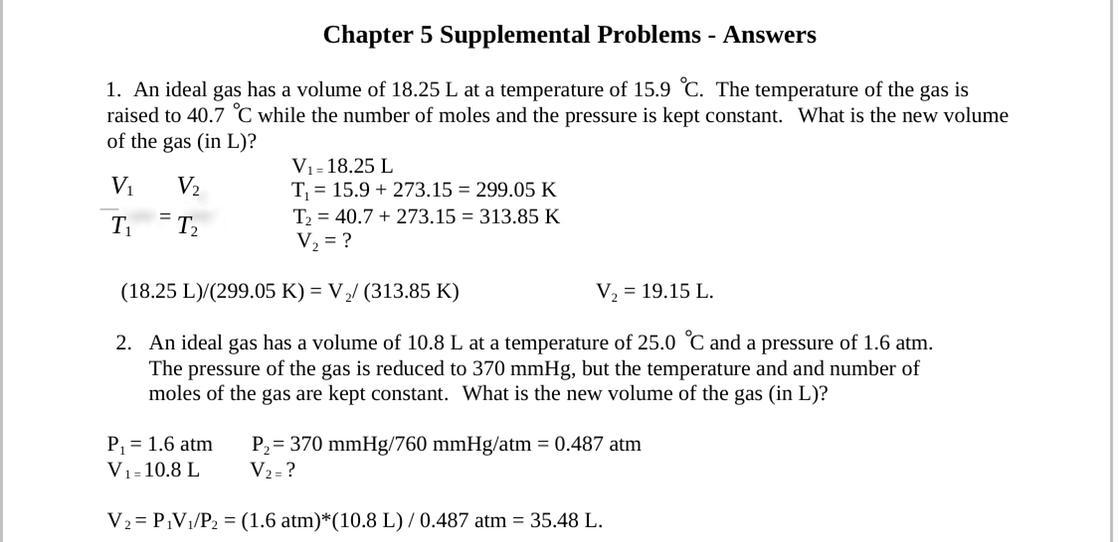
An ideal gas has a volume of 18.25 L at a temperature of 15.9 °C. The temperature of the gas is raised to 40.7 °C while the number of moles and the pressure of the gas are kept constant. What is the new volume of the gas (in L)?
B: An ideal gas has a volume of 10.8 L at a temperature of 25.0 °C and a pressure of 60 atm. The pressure of the gas is reduced to 370.0 mmHg, but the temperature and number of moles of the gas are kept constant. What is the new volume of the gas (in L)?
Answers
Answer:
V₂ = V₁ / T₁ * T₂ . If you prefer to set the final volume and want to estimate the resulting temperature, then the equation of Charles' law changes to: T2=T1/T1 multiplied by v^2.
Calcium is element 20 in the Periodic Table, has a mass of 40 amu and forms a 2+ ionic species. The calcium ion therefore has a. 18 protons, 18 neutrons and 22 electrons b. 22 protons, 18 neutrons and 18 electrons c. 20 protons, 20 neutrons and 18 electrons d. 18 protons, 20 neutrons and 20 electrons e. 20 protons, 18 neutrons and 20 electrons 1. In the following expression a∼1/b, what is the relationship between the components a and b ? a. Direct proportion b. None of the above c. Exact equation d. Inverse proportion e. Proportionality constant
Answers
The calcium ion has 18 protons, 20 neutrons, and 20 electrons.
The relationship between the components a and b is Inverse proportion.
The calcium ion (Ca2+) has a 2+ charge, indicating that it has lost 2 electrons from its neutral state. To determine the number of protons, neutrons, and electrons in the calcium ion, we need to consider its atomic number and mass.
The atomic number of calcium is 20, which indicates that it has 20 protons. Since the calcium ion has a 2+ charge, it means it has lost 2 electrons. Therefore, the number of electrons in the calcium ion is 20 - 2 = 18.
The mass number of calcium is 40 amu, which represents the total number of protons and neutrons. Since the calcium ion has 20 protons, the number of neutrons can be calculated as 40 - 20 = 20.
So, the correct option is: d. 18 protons, 20 neutrons, and 20 electrons
In the expression a∼1/b, the relationship between the components a and b is an inverse proportion. This means that as the value of a increases, the value of b decreases, and vice versa. The symbol ∼ represents the proportional relationship between a and 1/b, indicating that they are inversely related. Therefore, the correct answer is: Inverse proportion
To know more about calcium , click here, https://brainly.com/question/32135261
#SPJ11
What number need to go in front of ty HCI to balance the equation?
3H2 + 3Cl2 - ?HCI
Answers
As long as we have 3 in front of the compound so u have to multiple it by the number of the elements that are in the compound
We have 6 H
And 6Cl
So we need 6 HCl to balance the equation.
Hope that it helps
Earths system analysis graphic organizer
Answers
Answer:
attached
Explanation:

Draw the correct Lewis structure of this molecule by placing atoms on the canvas and connecting them with bonds. Include all hydrogen atoms and lone pairs of electrons.
Answers
Answer:
hello your question is incomplete attached below is the complete question
answer : attached below
Explanation:
The Lewis structure given in the question is incorrect attached below is the correct Lewis structure.
Lewis structure of a molecule is the representation of the valence electrons in a molecule


elements are organized on the periodic table according to
Answers
Answer:
atomic number
Explanation:
The periodic table is a table that lists all of the chemical elements in order of atomic number, starting with hydrogen and ending with oganesson. The number of protons in the nucleus of an atom of a certain element is its atomic number.
Have a good day :)
Please may someone help me with the bottom bit!

Answers
Precipitate definition (Entry 2 of 3) 1: a substance that has been physically or chemically isolated from a solution or suspension, typically in the form of an insoluble crystalline or amorphous solid. 2: a byproduct, outcome, or result of a procedure or action. adjective. precipitate.
What is an example of precipitate in chemistry?Precipitation Illustration
Silver chloride will solidify out of solution when silver nitrate and sodium chloride are combined in water. Silver chloride is the precipitate in this instance.
What does precipitate represent?Solids are created by the process of precipitation after two chemicals react. The downword () arrow symbol is used to represent the production of precipitate in this type of reaction.
What two types of precipitate are there?It happens between the reactant ions in the aqueous solutions that give rise to the material. Complete response: Precipitation includes the following: snow, sleet, hail, and rain.
What color is precipitate?An excess of the light blue precipitate dissolves to create an inky transparent solution. A little amount of sodium hydroxide solution and subsequently an excessive amount are added to solution W. There develops an insoluble white precipitate.
To Learn more About Precipitate, Refer:
https://brainly.com/question/1783904
#SPJ13

Help me with this please

Answers
Answer:
–253.5 °C
Explanation:
We'll begin by calculating the number of mole in 6 g of CO₂. This can be obtained as follow:
Molar mass of CO₂ = 12 + (2×16)
= 12 + 32
= 44 g/mol
Mass of CO₂ = 6 g
Mole of CO₂ =.?
Mole = mass / molar mass
Mole of CO₂ = 6 / 44
Mole of CO₂ = 0.136 mole
Next, we shall convert 225 mL to L.
1000 mL = 1 L
Therefore,
225 mL = 225 mL × 1 L / 1000
225 mL = 0.225 L
Next, we shall determine the temperature of the gas. This can be obtained as follow:
Pressure (P) = 0.855 atm
Volume (V) = 0.225 L
Number of mole (n) = 0.136 mole
Gas constant (R) = 0.0821 atm.L/Kmol
Temperature (T) =?
PV =nRT
0.855 × 0.255 = 0.136 × 0.0821 × T
0.218025 = 0.0111656 × T
Divide both side by 0.0111656
T = 0.218025 / 0.0111656
T = 19.5 K
Finally, we shall convert 19.5 K to degree celsius (°C). This can be obtained as follow:
T(°C) = T(K) – 273
T(K) = 19.5 K
T(°C) = 19.5 – 273
T(°C) = –253.5 °C
Therefore, the temperature of the gas is –253.5 °C
1: What is the partial pressure (atm) of nitrogen gas in a 2.50−L container into which 56 g of each nitrogen and carbon dioxide gases are added at 25∘
C ? A) 39,00 B) 39.10 C) 17.90 D) 19.55 E) 15200 Q2: Calculate the root mean square speed (m/s) of an A2 molecule (atomic mass =6amu ) at 0∘ C. A) 1506 B) 1.135×10 6
C) 1065.0 D) 1065.3 E) 753
Expert Answer
Answers
In a 2.50-L container with 56 g each of nitrogen and carbon dioxide gases added at 25°C, the partial pressure of nitrogen gas is 4.89 atm. The root mean square speed of an A₂ molecule at 0°C is approximately 8.65 m/s. None of the given options are correct.
Q1. We need to find the partial pressure (atm) of nitrogen gas in a 2.50−L container into which 56 g of each nitrogen and carbon dioxide gases are added at 25°C. Let's begin:
56g of N₂ will be equal to moles of N₂ = (56/28) = 2 mol N₂
56g of CO₂ will be equal to moles of CO₂ = (56/44) = 1.27 mol CO₂
Total moles of gas = 2 + 1.27 = 3.27 moles
Molar mass of N₂ = 28 g/mol
Ideal Gas equation = PV = nRT
Partial pressure of N₂ = P_N₂ = (n_N₂ * RT) / V
Where,
n_N₂ = number of moles of N₂ = 2 mol
R = gas constant = 0.08206 L atm K⁻¹ mol⁻¹
T = temperature in kelvin = (25 + 273.15) K = 298.15 K
V = volume of container = 2.50 L
Substituting the values in the formula, we get:
P_N₂ = (2 mol * 0.08206 L atm K⁻¹ mol⁻¹ * 298.15 K) / 2.50 L
P_N₂ = 4.89 atm
Therefore, the partial pressure (atm) of nitrogen gas in a 2.50−L container into which 56 g of each nitrogen and carbon dioxide gases are added at 25°C is 4.89 atm.
None of the given options are correct.
Q2. We need to find the root mean square speed (m/s) of an A₂ molecule (atomic mass =6amu ) at 0°C. Let's begin:
The root mean square speed (v rms) of an ideal gas is given by the formula:
v rms = √(3RT / M)
Where,
R = gas constant = 8.314 J/mol K
T = temperature in kelvin = 0 + 273.15 K = 273.15 K
M = molar mass of the gas = 6 g/mol = 6 / 1000 kg/mol = 0.006 kg/mol
Substituting the values in the formula, we get:
v rms = √(3 * 8.314 J/mol K * 273.15 K / 0.006 kg/mol)
v rms = √(3 * 8.314 J/mol K * 273.15 K / 6 * 10⁻³ kg/mol)
v rms = √(74.81)
v rms = 8.65 m/s (approx.)
Therefore, the root mean square speed (m/s) of an A₂ molecule (atomic mass =6amu ) at 0°C is 8.65 m/s (approx.).
None of the given options are correct.
To know more about root mean square speed, refer to the link below:
https://brainly.com/question/30759623#
#SPJ11
Complete Question:
Q24: What is the partial pressure (atm) of nitrogen gas in a 2.50-L container into which 56 g of each nitrogen and carbon dioxide gases are added at 25°C ? A) 39.00 B) 39.10 C) 17.90 D) 19.55 B) 15200
Q25: Calculate the root mean square speed (m/s) of an A2 molecule (atomic mass =6amu) at 0°C. 4) 1506 B) 1.135×10⁶ C) 1065.0 D) 1065.3 E) 753
10. What is the Kinetic Energy of a 100 kg object that is moving with a speed of 13.5 m/s?
Answers
Answer:
\(100 \times 13.5 = ikw n bahaa\)
IKAW na mag tayms
What mass of ZnO is formed when 29.2 g of MoO3is reacted with 17 g of Zn
Answers
21.16 g. Balance the equation, as stated. Mo2O3 + 3 ZnO = 3 Zn + 2 MoO3. and have their molar masses ready. Zn - 65.38 MoO3 - 143.96 Mo2O3 - 239.92.
What is Limiting Reagent?When a chemical reaction is complete, the limiting reagent—also known as the limiting reactant or limiting agent—is the reactant that has been completely consumed. As the reaction cannot proceed without this reagent, the amount of product that can be produced is constrained. Excess reagents or excess reactants are any reagents that are present in amounts greater than those necessary to cause a reaction with the limiting reagent (sometimes abbreviated as "xs"). Although the amount of product produced when the limiting reagent interacts entirely is defined as the theoretical yield, the limiting reagent must be determined in order to calculate the percentage yield of a reaction. Considering the reaction's description in the balanced chemical equation.
To know more about above topic visit:
https://brainly.com/question/26905271
#SPJ1