THIS IS TIMED!! PLEASE HELP A student has about 250 mL of the liquid acetone. She wants to get a precise and accurate measure of the actual volume of the liquid without losing any. Which of these would be the best instrument to use. 500 mL graduated cylinder (±1mL) 100 mL graduated cylinder (±0.5mL) 250 mL beaker (±5mL) 500 mL beaker (±7mL)
Answers
Answer:
500 mL graduated cylinder (±1mL)
Explanation:
Several laboratory glasswares are used in the laboratory, which serves different functions. They include: beakers, volumetric flask, Erlenmeyer flask etc. Beakers are flat-based glasswares used majorly for storing and mixing chemical solutions. Some beakers are also used to measure liquids.
However, graduated cylinders are tall flasks that has graduated markings on it, hence, it is majorly used for measuring liquids. According to this question, a student wants to get a precise and accurate measure of the actual volume of acetone liquid, which is about 250mL.
To do this, the best instrument she can use is a 500 mL graduated cylinder (±1mL). This is because the graduated cylinder holds enough space to contain the liquid and measure it with a level of accuracy better than beakers.
Related Questions
1. Write the IUPAC names for the following 1.1 1.2 N 1.3 O NO2 x Y ·0 OH 5
Answers
1. The IUPAC name of N is nitrogen.
2. Nitrogen dioxide
3.The IUPAC name of O is oxygen
4.The IUPAC name of OH is hydroxyl.
The IUPAC name of ·0 is a radical. It is commonly found in organic chemistry and plays an important role in many reactions.
IUPAC names for the given compounds are:1.1. N: Nitrogen
The IUPAC name of N is nitrogen.
It is a non-metal and belongs to group 15 in the periodic table. It has an electronic configuration of 1s2 2s2 2p3.1.2. NO2: Nitrogen dioxide
Explanation: NO2 is a chemical compound that is formed by the combination of nitrogen and oxygen. It is a reddish-brown gas that has a pungent odor.
The IUPAC name of NO2 is nitrogen dioxide.1.3. O: Oxygen
Explanation: The IUPAC name of O is oxygen.
It is a non-metal and belongs to group 16 in the periodic table. It has an electronic configuration of 1s2 2s2 2p4.
X: UnknownExplanation: No IUPAC name can be given to an unknown compound as the structure and composition are not known.
Y: Hydroxyl Explanation: The IUPAC name of OH is hydroxyl.
It is a functional group that is composed of an oxygen atom and a hydrogen atom (-OH). It is commonly found in alcohols and phenols. ·0: RadicalExplanation: A radical is a molecule or an ion that contains an unpaired electron.
for more question on electronic configuration
https://brainly.com/question/26084288
#SPJ8
Note: The complete question is given below
Provide the IUPAC names for the following compounds:
\(CH_3CH_2CH(CH_3)CH_2CH_2CH_2CH_3\)
C6H5CH(CH3)2
H2NCH2CH2CH2CH2CH2NH2
CH3CH2CH2CH2CH2OH
CH3CH2CH2CHOHCH3
what volume in liters of 0.5 m cacl2 solution can be made using 1200.0 g cacl2
Answers
Answer:
21.62 Liters
Explanation:
To determine the volume of a 0.5 M CaCl2 solution that can be made using 1200.0 g of CaCl2, you will need to use the molar mass of CaCl2 and the definition of molarity.
The molar mass of CaCl2 is:
CaCl2 = 1 x 40.08 g/mol (molar mass of Ca) + 2 x 35.45 g/mol (molar mass of Cl)
CaCl2 = 110.98 g/mol
To calculate the number of moles of CaCl2 in 1200.0 g, you can divide the mass by the molar mass:
moles of CaCl2 = 1200.0 g / 110.98 g/mol
moles of CaCl2 = 10.81 mol
Since molarity is defined as the number of moles of solute per liter of solution, you can calculate the volume of a 0.5 M CaCl2 solution that can be made using 10.81 moles of CaCl2:
0.5 M = 0.5 moles of CaCl2 per liter of solution
Volume of solution = moles of CaCl2 / Molarity
Volume of solution = 10.81 mol / 0.5 mol/L
Volume of solution = 21.62 L
Therefore, you can make 21.62 L of a 0.5 M CaCl2 solution using 1200.0 g of CaCl2.
In the given reaction the cyanoacetate first treated with methoxide and then , slowly added to the aldehyde. Draw the structure of the major product: Select Draw More Erase CH;O CHzOH Rings
Answers
To draw the structure of the main product, we need to start with the aldehyde and add the cyanoacetate group. The structure of the main products is:
H
|
C-O
|
H
CH3O-
|
C≡N-C-O-CH3
This reaction is an example of a Michael reaction in which a cyanoacetate enolate (formed by treatment with methoxide) reacts with an aldehyde to form a β-ketoester.
The reaction occurs via an electrophilic addition mechanism, with the cyanoacetate enolate acting as the nucleophile and the aldehyde as the electrophile. The resulting product is a β-ketoester characterized by the presence of a ketone group (C=O) and an ester group (C-O-C) in the same molecule.
Read more about organic chemistry at:
brainly.com/question/17567426
#SPJ4
A solution contains 1.817 mg of CoSO4 (155.0 grams/mole) per mL. Calculate the volume (in mL) of 0.009795 M Zn2 needed to titrate the excess complexing reagent after the addition of 70.00 mL of 0.009005 M EDTA to a 20.00 mL aliquot of the Co2 solution.
Answers
Answer:
85.952 ml \(Zn^2^+\) needed to titrate the excess complexing reagent .
Explanation:
Lets calculate
After addition of 80 ml of EDTA the solution becomes = 20 + 70 = 90 ml
As the number of moles of \(CoSO_4\) =\(\frac{Given mass }{molar mass}\)
=\(\frac{1.817}{155}\)
=0.01172
Molarity = \(\frac{no. of moles}{volume of solution}\)
=\(\frac{0.01172}{20}\)
=0.000586 moles
Excess of EDTA = concentration of EDTA - concentration of CoSO4
= 0.009005 - 0.000586
= 0.008419 M
As M1V1 ( Excess of EDTA ) = M2V2 \((Zn^2^+)\)
\(0.008419\times100ml=0.009795\times V2\)
\(V2=\frac{0.008419\times100}{0.009795}\)
V2 =85.952 ml
Therefore , 85.952 ml \(Zn^2^+\) needed to titrate the excess complexing reagent .
Heat in the amount of 100 kJ is transferred directly from a hot reservoir at 1400 K (TH) to a cold reservoir at 600 K. Calculate the entropy change of the two reservoirs. The entropy change of the two reservoirs is kJ/K. Is the increase of entropy principle satisfied
Answers
Answer:
ΔS = - 0.125 Kj/K decreased entropy
Explanation:
From ΔH = TΔS => ΔS = ΔH/T
= 100Kj/(600K - 1400K) = - 188Kj/800K = - 0.125 Kj/K
Entropy in the cooler flask would have 'decreased' (i.e.; more ordered state) at 600K.
A chemist determines that a substance is composed of 30.4% nitrogen by mass and 69.6% oxygen by mass. The molar mass of the compound is 230.5 g/mol.
Answers
A chemist determines that a substance is composed of 30.4% nitrogen by mass and 69.6% oxygen by mass. The molar mass of the compound is 230.5 g/mol, the molecular formula is NO₂.
We must compute the empirical formula in order to ascertain the compound's chemical composition.
If we have 100 grams of the compound.
This suggests we have:
30.4 g of nitrogen
69.6 g of oxygen
Now, we have to convert the mass of each element to moles.
The molar mass of nitrogen (N) = 14.01 g/mol
the molar mass of oxygen (O) = 16.00 g/mol.
Number of moles of nitrogen (N):
2.17 mol
Number of moles of oxygen (O):
4.35 mol
The simplest whole-number ratio between the moles of nitrogen and oxygen must now be determined. To calculate the ratio, we divide both numbers by the smaller value.
Moles N / moles O = 2.17 mol / 2.17 mol = 1.00
Moles O / moles O = 4.35 mol / 2.17 mol = 2.00
The ratio is approximately N₁O₂.
We divide the subscripts by their greatest common divisor to obtain the simplest ratio, since we are looking for the empirical formula. The empirical formula is NO₂ since the greatest common divisor in this situation is 1.
The molecular formula of the compound is NO₂.
Learn more about molecular formula, here:
https://brainly.com/question/11203434
#SPJ1
anyone here know about the law of assumption?
Answers
Answer:
uhhh not really i don't even know what that is LOL
Heat flows from hotter objects to colder objects until it reaches a state where heat is no longer flowing. What have the objects reached when heat is no longer
flowing?
Answers
Answer:
It has reaced thermal equlibrum
How many grams of Aluminum Sulfate are produced when 4 g of Aluminum Nitrate react with 3 g of Sodium Sulfate?
Al(NO3)3 + Na2SO4 ---------> Al2(SO4)3 + NaNO3
Answers
3.21 grams of Aluminum Sulfate are got when 4 g of Aluminum Nitrate reacts chemcially with 3 g of Sodium Sulfate.
WHat is the balanced equation for this reaction? How many grams of Aluminum Sulfate are produced?The equation given is not balanced. Thus, when balanced the equation becomes:
2 Al(NO₃)₃ + 3 Na₂SO₄ → Al₂(SO₄)₃ + 6 NaNO₃
The molar mass of Al(NO₃)₃ is:
Al(NO₃)₃ = 1(Al) + 3(N) + 9(O) = 213 g/mol
The molar mass of Na₂SO₄ is:
Na₂SO₄ = 2(Na) + 1(S) + 4(O) = 142 g/mol
From the balanced equation, we can see that 2 moles of Al(NO₃)₃ react with 3 moles of Na2SO4 to produce 1 mole of Al₂(SO₄)₃. Therefore, we can calculate the number of moles of Al(NO₃)₃ and Na₂SO₄ that react:
Number of moles of Al(NO₃)₃ = 4 g / 213 g/mol = 0.0188 mol
Number of moles of Na₂SO₄ = 3 g / 142 g/mol = 0.0211 mol
From the balanced equation, we can see that 2 moles of Al(NO₃)₃ produce 1 mole of Al₂(SO₄)₃. Therefore, the number of moles of Al₂(SO₄)₃ produced is:
Number of moles of Al₂(SO₄)₃ = 0.0188 mol / 2 * 1 = 0.0094 mol
The molar mass of Aluminum Sulfate (Al₂(SO₄)₃) is:
Al₂(SO₄)₃ = 2(Al) + 3(S) + 12(O) = 342 g/mol
Therefore, the mass of Aluminum Sulfate produced is:
Mass of Al₂(SO₄)₃ = Number of moles of Al₂(SO₄)₃ * Molar mass of Al₂(SO₄)₃
= 0.0094 mol * 342 g/mol
= 3.21 g
Hence, 3.21 grams of Aluminum Sulfate are liberated when 4 g of Aluminum Nitrate change state with 3 g of Sodium Sulfate.
Learn more about balanced chemical equation here:
https://brainly.com/question/28294176
#SPJ1
URGENT!!! An unknown hydrate of CoCl₂ has been evaporated in a crucible. Given the following data, find the formula and name of the hydrate.
Mass of crucible: 12.090 g
Mass of hydrate before evaporation and crucible: 16.250 g
Mass of hydrate after evaporation and crucible: 12.424 g
Answers
From the given data, the name of the hydrated salt would be \(CoCl_2.83H_2O\).
Formula of hydrateThe formula of the hydrated salt can be determined using the empirical formula approach. That is, we will find the mole equivalent of the anhydrous salt and the water of hydration and then combine them into a single formula after dividing by the smallest mole.
First, we need to determine the mass of the anhydrous salt and the water of hydration.
Mass of crucible (x) = 12.090 g
Mass of hydrated salt + crucible (y) = 16.250 g
Thus, the mass of the hydrated salt can be determined by subtracting x from y.
Mass of hydrated salt = 16.250 - 12.090 = 4.16 g
Mass of hydrate + crucible after evaporating off the water (z) = 12.424 g
Mass of anhydrous salt = z - x
= 12.424 - 12.090
= 0.334 g
Mass of water = 4.16 - 0.334
= 3.826 g
Now, let's find the moles:
Molar mass of \(CoCl_2\) = 129.839 g/mol
Molar mass of water = 18.01 g/mol
Mole of \(CoCl_2\) = 0.334/129.839 = 0.00257 mol
Mole of water = 3.826/18.01 = 0.2124 mol
Dividing through by the smallest mole
\(CoCl_2\) = 0.00257 / 0.00257 = 1
water = 0.2124/ 0.00257 = 83
Thus, the formula of the hydrate would be \(CoCl_2.83H_2O\)
More on hydrate salts can be found here: https://brainly.com/question/16990374
#SPJ1
How does the availability of WATER affect the growth of plants and animals?
Answers
Answer:
all living things need water. no water, no life.
the male and female sex cells combine to form a
Answers
Answer:
zygote
Explanation:
In human beings, each gamete contains 23 chromosomes, half the number found in the other cells of the body. When the male and female gametes fuse, they become a zygote containing the full 46 chromosomes, half of which came from the father and half from the mother.
Answer:
zygote
Explanation:
In human beings, each gamete contains 23 chromosomes, half the number found in the other cells of the body. When the male and female gametes fuse, they become a zygote containing the full 46 chromosomes, half of which came from the father and half from the mother.
When 7.524 is rounded to 3 sig figs it will be
Answers
When 7.524 is rounded to 3 significant figures, it will be 7.52.
The process of changing a number to a nearby number with fewer significant digits is known as rounding.
Rounding can be done to the nearest integer, the nearest tenth, the nearest hundredth, and so on.
Here are some pointers on rounding numbers to a certain number of significant digits:If the digit following the last significant digit is less than 5, simply drop it and all following digits.
(round down)For example, 2.832 rounded to two significant digits is 2.8 since the 3 is followed by a 2 which is less than 5.
If the digit following the last significant digit is greater than 5, add 1 to the last significant digit, then drop all of the digits that follow it.
(round up)For example, 4.673 rounded to two significant digits is 4.7 since the 3 is followed by a 7 which is greater than 5.
If the digit following the last significant digit is exactly 5, the preceding digit is odd, and no other digits follow, increase the last significant digit by 1.
If the digit following the last significant digit is exactly 5, the preceding digit is even, and no other digits follow, simply leave the last significant digit alone.
For example, 2.875 rounded to two significant digits is 2.9 since the 5 is followed by an odd number, which means that the 8 should be rounded up, while 2.765 rounded to two significant digits is 2.8 since the 5 is followed by an even number, which means that the 6 should be left alone.
For more such questions on rounding
https://brainly.com/question/17396482
#SPJ8
22.What number should be placed in front of the H2O in the reaction below?2 Fe(OH)3 ---> Fe2O3 + ___ H2OSelect one:a. 1b. 2c. 3d. 6
Answers
Answer
C. 3
Explanation
On the left side there are 6 oxygens and 6 hydrogens. Then on the right side there should be the same number of atoms, if we add 3 infront of H2O, there will be 6 oxygens and 6 hydrogens.
297.85
Question 2
4 pts
If a sample of gas occupies 23.5 mL at 315 K and 14.8 atm of pressure, what volume will it occupy at 415 K and
12.3 atm?
Give your volume in ml, but do not include the units in the answer. Do not use scientific notation.
Question 3
4 pts
Answers
Answer:
37.25
Explanation:
\(P_1\) = Initial pressure = \(14.8\ \text{atm}\)
\(P_2\) = Final pressure = \(12.3\ \text{atm}\)
\(V_1\) = Initial volume = \(23.5\ \text{mL}\)
\(V_2\) = Final volume
\(T_1\) = Initial temperature = \(315\ \text{K}\)
\(T_2\) = Final temperature = \(415\ \text{K}\)
From ideal gas law we have
\(PV=nRT\)
\(\Rightarrow PV\propto \dfrac{1}{T}\)
So
\(\dfrac{P_1V_1}{T_1}=\dfrac{P_2V_2}{T_2}\\\Rightarrow V_2=\dfrac{P_1V_1T_2}{T_1P_2}\\\Rightarrow V_2=\dfrac{14.8\times 23.5\times 415}{315\times 12.3}\\\Rightarrow V_2=37.25\ \text{mL}\)
The final volume is \(37.25\ \text{mL}\)
ASAP PLS
A 18.7 g piece of aluminum (which has a heat capacity of 0.89 JPC-g) is
heated to 82.4°C and dropped into a calorimeter containing water
(specific heat capacity of water is 4.18 J/gºC) initially at 22.3°C. The final
temperature of the water is 24.3°C. Ignoring significant figures, calculate
the mass of water in the calorimeter. *

Answers
Answer:
think I did this before and its V
once energy is applyied to cause a object to vibrate a sound occours
Answers
Answer:
its true
Explanation:
Answer:
true
Explanation:
you stop on ground, ground vibrates and makes sound
Look at the attachment below.

Answers
Sally is wrong because copper is less electropositive than hydrogen, thus, can not displace hydrogen from dilute acids.
The reactions to prepare copper (ii) chloride are:
the chlorination of copper sulfide at a high temperature
reaction of copper (ii) oxide with dilute hydrochloric acid
The equations of the given reactions are as follows:CuS + Cl₂ ---> CuCl₂ + SCuO + 2HCl ----> CuCl₂ + H₂O
What are reactive metals?Reactive metals are metals that readily give up their electrons to form positive ions.
Reactive metals displace hydrogen from dilute acids. They are found in group 1A and 2A of the periodic table. Copper is not a reactive metal and will not displace hydrogen from acids.
Learn more about reactive metals at: https://brainly.com/question/20273277
#SPJ1
Sally is wrong because copper chloride is not made from the reaction of copper and dilute hydrochloric acid.
2. Copper (ii) chloride can be prepared as follows:
reacting copper (ii) oxide with dilute hydrochloric acidsingle replacement reaction of copper sulfide and chlorine gas at a high temperature3. the equations of the reaction are:
CuO + 2HCl ----> CuCl₂ + H₂OCuS + Cl₂ ---> CuCl₂ + SWhat are single replacement reactions?Single replacement reactions are reactions in which a more reactive atom replaces another atom in a compound.
An example of a single replacement reaction is the reaction of chlorine gas with copper sulfide at high temperatures to form copper chloride.
Learn more about single replacement reaction at: https://brainly.com/question/20216315
#SPJ1
I need help I don’t understand this is hitting

Answers
Reagents that are entirely consumed by a chemical reaction are known as limiting reagents.
Thus, They are additionally known as limiting reactants or limiting agents. A predetermined quantity of reactants are necessary for the reaction to be completed, according to the stoichiometry of chemical reactions.
In the aforementioned reaction, 2 moles of ammonia are created when 3 moles of hydrogen gas react with 1 mole of nitrogen gas.
In most cases, this reactant dictates when the reaction will end. The reaction stoichiometry can be used to determine the precise quantity of reactant that will be required to react with another element. The limiting agent is determined by the mole ratio rather than the mass of the reactants.
Thus, Reagents that are entirely consumed by a chemical reaction are known as limiting reagents.
Learn more about Chemical reaction, refer to the link:
https://brainly.com/question/22817140
#SPJ1
2C2H2(g) + 502(g) → 4C02(g) + 2H20(g) reaction type?
Answers
Answer:
The answer is combustion.
Determine the mass of the following samples
3 moles of Mg
4.2 moles of NaCI
Answers
Answer:
See below
Explanation:
From periodic chart
Mg = 24.305 gm per mole
24.305 * 3 = 72.915 gm
Na = 22.989
Cl = 35.45 summed = 58.439 gm per mole
58.439 * 4.2 = 245.4438 gm
consider the compounds shown, which are all acids of group 7 atoms. in general, bond strength down a group in the periodic table, as the size of the atom bonded to h increases. acid strength therefore down the group.
Answers
In general, bond strength down a group in the periodic table, as the size of the atom bonded to H increases, acid strength, therefore, increases down the group.
Electronegativity is a measure of the tendency of an atom to attract electron density or electrons towards itself.
In a polar covalent bond, electrons are shifted toward the more electronegative atom; therefore, the more electronegative atom is the one with the partial negative charge. The more polarized the electron distribution and the larger the partial charges of the atoms, the greater the difference in electronegativity.
The absolute value of the difference in electronegativity of two bonded atoms provides a rough measure of the polarity to be expected in the bond and the bond type. The bond is covalent and nonpolar when the difference is very small or zero. The bond is polar covalent or ionic when it is large.
To learn more about bond strength,
brainly.com/question/21557710
#SPJ4
The correct question is:
Consider the compounds shown, which are all acids of group 7 atoms. In general, bond strength down a group in the periodic table, as the size of the atom bonded to h increases. What happens to acid strength down the group?
Some students want to investigate the relationship between the pressure and volume of a gas. They take a sealed, thin-walled metal can full of air, place it in a vat of liquid nitrogen, and observe that the can collapses. Another student says that their procedure is a better demonstration of the effect of temperature on another property of the gas. Which of the following properties of the gas does the procedure best relate to temperature?
a. Volume.
b. Pressure.
c. The amount of gas.
d. The universal gas constant.
Answers
The ideal gas equation allows finding that the best variable for the temperature experiment is:
a. Pressure
b. Volume
Ideal gases are gases that do not have any interaction between their molecules, they are described by the relationship
P V = n R T
Where P is the pressure, V the volume, n the number of moles, R the ideal gas constant and T the absolute temperature.
In the problem the students want to study the effect of temperature with ideal gases equation we see that pressure and volume are directly proportional to temperature.
Of these two properties, the easiest to measure is pressure, keeping the volume constant.
In conclusion using the ideal gas equations allows finding that the best variable for the temperature experiment are :
a. Pressure
b. volume
Learn more here: brainly.com/question/3491421
Water with a salinity of 20 g/L has a density of
_kg/m
3
Answers
Answer:
20 kg/m3
Explanation:
(The question is in the photo Sorry lol) A sample of a gas occupies 1234 mL at 237°C and 740 torr. At what temperature would it occupy 1023.4 mL if the pressure is kept constant?Answer in units of °C.✨ Please leave the numbers after the decimal in the final answer

Answers
Okay so if the temperature is constant, and you want either V or T then it is Charles gas law:
\(\frac{V_1}{T_1}\text{ = }\frac{V_2}{T_2}\)We are given the following:
V1 = 1234 mL
T1 = 237 degrees celsius
V2 = 1023.4 mL
T2 = ?
Since we want the answer in degrees celsius, we do not need to covert to kelvin.
T2 = (T1V2)/V1
T2 = (237 x 1023.4)/1234
T2 = 196.5525122 degrees celsius
Aluminum is an element. Which of the following best describes the smallest particle of aluminum that retains all the properties of aluminum?
A. Amolecule.
B. an atom
C. a proton.
D. an electron
Answers
Answer:
molecule
Explanation:
Answer:
b
Explanation:
protons and electrons can be in all atoms and it's not A because a molecule can contain aluminum it is not the smallest. The answer is B because an atom has all of the properties of aluminum and it can't get smaller without losing its properties.
Is this right:) please tell me
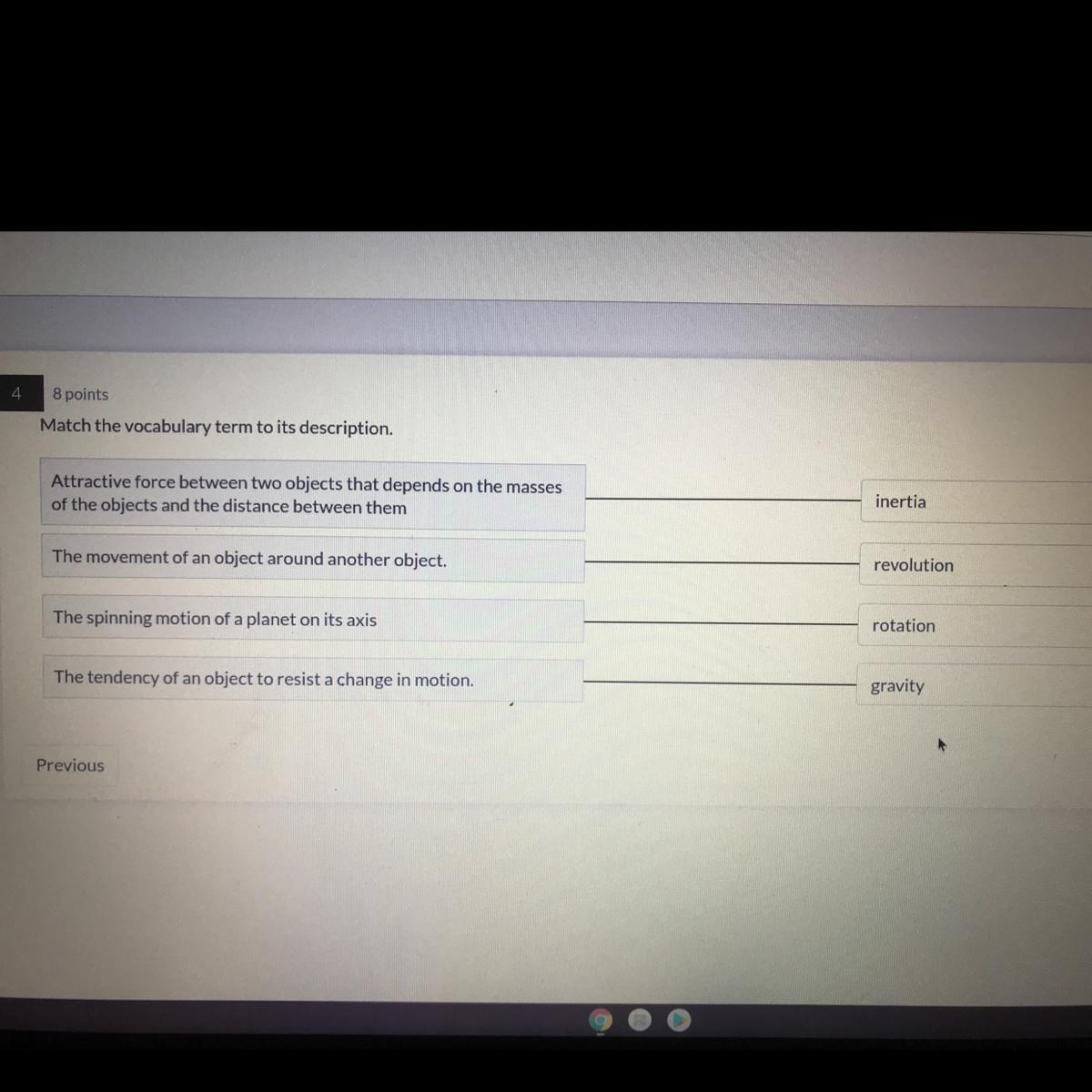
Answers
Answer:
I think that:
The tendency of an object to resist changes in motion: inertia
Attractive force:gravity
And everything else is right
Explanation:
I am not a physicist
But I passed physics with an A
After running, Ollie’s blood pressure was high. His nervous system sensed this and sent a message to decrease heart rate and blood flow.
Answers
Answer:
Circulatory
Explanation:
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
Americans drink 5,604,000 gallons of sweet tea each day. How many liters is
that? (1 gal = 3.79 L)
Answers
5604000gallon =21239160 liters