Answers
Answer:
The nitrogen atom has 5 electrons in the outermost shell, so it can accept 3 electrons to fulfil the octet structure. Therefore the valency of nitrogen is 3.
Related Questions
Critically discuss the refusal/unwillingness of some individuals to answer questions to put them by authorized Stats SA officials
Answers
People refuse to answer questions from authorized Stats SA officials because they don't want to reveal confidential information.
What does official stats refer to?An official Stats is an official who has the function of asking citizens for different information in order to establish general statistics for all citizens.
Why don't people answer your questions?Some people refuse to answer questions out of mistrust, because they don't want to share personal information.
Learn more about personal information in: https://brainly.com/question/451424
#SPJ1
What type of energy does a skier stopped at the top of a hill have because of
his or her position?
A. Kinetic energy
B. Gravitational potential energy
C. Heat energy
D. Chemical energy
Answers
Answer:
B
Explanation:
Which of the following aqueous solutions are good buffer systems?
a. 0.34 M calcium iodide + 0.22 M sodium iodide.
b. 0.27 M ammonia + 0.38 M ammonium nitrate.
c. 0.27 M nitric acid + 0.18 M sodium nitrate.
d. 0.18 M hydrofluoric acid + 0.14 M hydroiodic acid.
e. 0.14 M calcium hydroxide + 0.28 M calcium chloride.
Answers
Answer:
b. 0.27 M ammonia + 0.38 M ammonium nitrate.
Explanation:
Hello there!
In this case, according to the given information, it turns out possible for us to bear to mind the fact that buffest must be prepared by using either of the following pairs:
weak acid/conjugate base
weak base/conjugate acid
So that the pH might be set constant. In such a way, since a. shows two salts, c. a strong acid with a neutral base, d, shows two acids and e. a strong base with a neutral base, we infer the correct buffer is b. 0.27 M ammonia + 0.38 M ammonium nitrate because it has a weak base (ammonia) and its conjugate acid, ammonium.
Regards!
Macmillan Learning Determine the formal charge on each atom in the structure. H H-B-H H What is the overall charge on the structure? -2 +1 Answer Bank +2 +3 -3 -4 +4 0
Answers
The overall charge on the structure is negative one (-1).
The central boron atom in the structure is bonded to two hydrogen atoms. Boron has three valence electrons, and it has formed only two bonds, so it has a formal charge of +1.
Each of the hydrogen atoms has one valence electron, and each is bonded to the boron atom, so each hydrogen atom has a formal charge of -1. The sum of the formal charges in the structure is equal to the charge of the ion, which is -2. Adding up the formal charges of the atoms, we get:
B: +1
H: -1 (two times)
Overall charge = sum of formal charges = +1 - 1 - 1 = -1
Therefore, the overall charge on the structure is negative one (-1).
Learn more about formal charge, here:
https://brainly.com/question/11723212
#SPJ1
Elements on the periodic table are arranged into vertical columns called groups. Which of the following is true of the elements in a group?
O All the elements in a group have the same mass.
Elements in a group have the same number of neutrons.
Elements in a group have similar properties.
O Elements in a group have the same number of protons.
Answers
Answer:
Elements in a group have the some number of protons.
Explanation: was the correct answer when i took a test
Elements on the periodic table are arranged into vertical columns called groups.Elements in a group have the same number of protons.is true of the elements in a group. Therefore, option D is correct.
What is periodic table ?The chemical elements are arranged in rows and columns in the periodic table, sometimes referred to as the periodic table of the elements. It is often used in physics, chemistry, and other disciplines, and is frequently regarded as a symbol of chemistry.
The elements are organized in what is known as the periodic table. Columns and rows are used to organize the table.
The horizontal rows are referred to as "periods," while the vertical columns are known as "groups." The elements that appear in identical groupings (columns) share comparable characteristics.
Thus, option D is correct.
To learn more about the periodic table, follow the link;
https://brainly.com/question/11155928
#SPJ2
PLEASE HELP!!!
How many calories are in 4,180 joules?
Answers
Answer:
To convert joules to calories, you can use the conversion factor:
1 calorie = 4.184 joules
To find out how many calories are in 4,180 joules, divide the given value by the conversion factor:
4,180 joules / 4.184 joules per calorie = 0.9 calories (approximately)
Therefore, there are approximately 0.9 calories in 4,180 joules.
Lewis Structure Practice (No Links Or I will Report)

Answers
Answer:
.. ..
:O = C - Cl:
.. | ..
:Cl:
..
How many moles of zinc, Zn, are in 1.31×1024 Zn atoms?
Answers
Answer:
=7.89013× 10^47 moles of zinc
Explanation:
1 atom contains 6.023×10^23 moles.
1.31×10^24 atoms of zinc contain6.023×10^24×1.31×10^.24
a
A 49.8 mL sample of gas in a cylinder is warmed
from 18°C to 83 °C.
what is it’s volume at the final temperature? (Assume constant pressure)
Answers
Using Charles law
\(\\ \sf\longmapsto V_1T_2=V_2T_1\)
\(\\ \sf\longmapsto V_2=V_1T_2\div T_1\)
\(\\ \sf\longmapsto V_2=\dfrac{49.8(356)}{291}\)
\(\\ \sf\longmapsto V_2=\dfrac{17728.8}{291}\)
\(\\ \sf\longmapsto V_2=60.9mL\)
The diagram below left shows a box containing gas molecules at 45 degrees Celsius and 1.25 atm pressure. The piston is free to move. Giving brainliest

Answers
The temperature and pressure of the left box is 250c (298 K), and 1 atm pressure.
How to solve thisThe right box is at standard temperature and pressure.
Standard temperature and pressure is 00c (273.15 K) and 1 atm.
Hence, the pressure is the same, therefore the position is piston will be the same as the left figure.
At constant pressure, volume is directly proportional to absolute temperature.
\(V \propto T .\)
Now, as the temperature at the right box is less than 250c that of the left box, hence the volume decreases significantly.
The arrangement of molecules in the right box will be closer.
A diagram of the right box is given below.
Read more about atm pressure here:
https://brainly.com/question/19587559
#SPJ1

I need help on #10 please

Answers
Answer:
Na,Ba,Ti,on their placement on the periodic table...
Why is the regression equation not exactly y = 100 • 0.5n?
Answers
Answer:
Radioactive decay is a random event.
Explanation:
on edge
Answer:
Radioactive decay is a random event.
Explanation:
Edge 2020
60 points!! Look at picture please don’t troll
Answers
Help me please very confused?
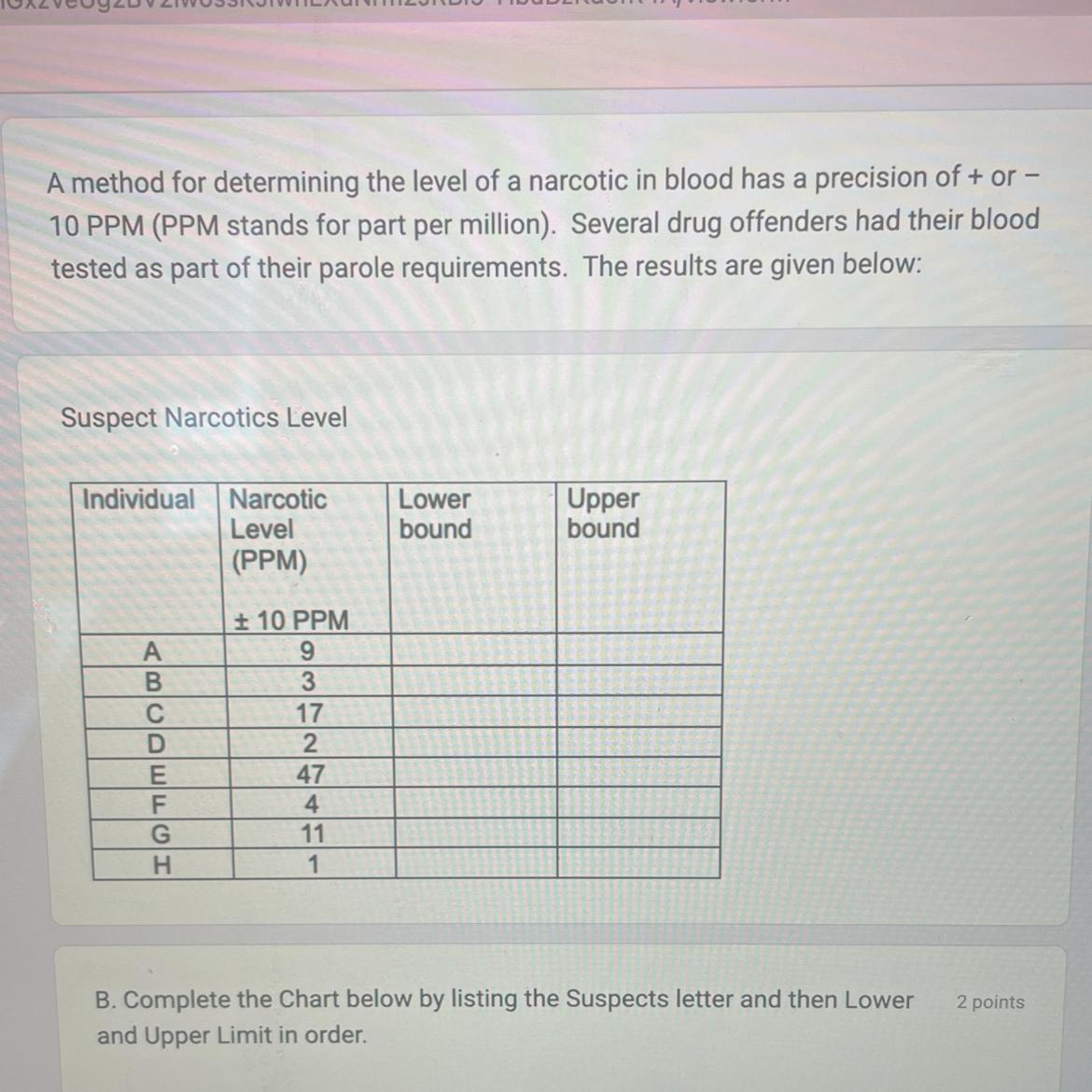
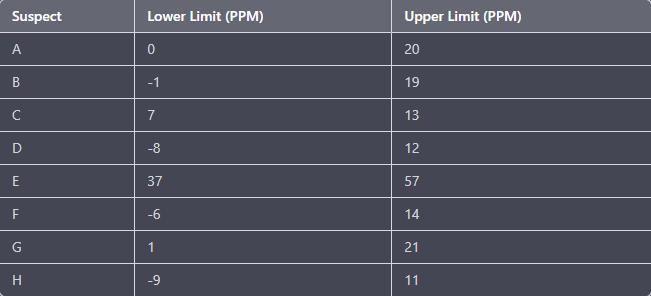
Answers
Answer:
Your answer is on the attached image. I hope this helps!
Consider marking Brainliest!
Do grasshoppers have wings
Answers
Answer: yes
Explanation: I feed grasshoppers to frogs
what is the answers to this someone pls help

Answers
Answer:
The nuclide formed by the β decay of 26Al is 26Mg.
Mark my answer as brainliest! this was a difficult one
The names and chemical formulas of four substances are shown in the chart.
Carbonic acid- H2CO3
Nitric acid- HNO3
Phosphoric acid- H3PO4
Sulfuric acid- H2SO4
Which substance listed in the chart is made up of the most atoms?
A. Sulfuric acid
B. Phosphoric acid
C. Nitric acid
D. Carbonic acid
Answers
Among the presented substances, Phosphoric acid - H₃PO₄ is the one made up of most atoms.
What is atomicity?Atomicity refers to the number of atoms of each element in a compound. It is given by the corresponding subscripts.
We have 4 substances. To calculate the number of atoms in each one, we will sum the number of atoms that form them.
Carbonic acid - H₂CO₃H + C + O = 2 + 1 + 3 = 6 atoms
Nitric acid - HNO₃H + N + O = 1 + 1 + 3 = 5 atoms
Phosphoric acid - H₃PO₄H + P + O = 3 + 1 + 4 = 8 atoms
Sulfuric acid - H₂SO₄H + S + O = 2 + 1 + 4 = 7 atoms
Among the presented substances, Phosphoric acid - H₃PO₄ is the one made up of most atoms.
Learn more about atoms here: https://brainly.com/question/13453032
Answer:
B
Explanation:
Phosphoric acid
You perform a titration between Sodium Hydroxide (NaOH) and Hydrochloric Acid (HCl). NaOH is in the burette and the HCl is in the Erlenmeyer Flask. 41.8 mL of 0.5 M NaOH was added to 37mL of HCl, what is the concentration of the HCl? Give your answer to 2 decimal places.
Answers
From the calculation, the concentration of the HCl is obtained as 0.56 M.
What is titration?The term titration has to do with the reaction between an acid and a base to yield salt and water only. The equation of the reaction is;
NaOH + HCl ----> NaCl + H2O
Given the titration formula;
CAVA/CBVB = NA/NB
CAVANB = CBVBNA
CA = CBVBNA/VANB
CA = 0.5 M * . 41.8 mL * 1/37mL * 1
CA = 0.56 M
Learn more about titration:https://brainly.com/question/2728613
#SPJ1
what is the maximum amount of KNO3 that can be dissolved in 100 g of 50 C water ? A. 15g B. 36g C. 84g D. 100g

Answers
From the solubility curve, it is clear that 84 g of potassium nitrate can be dissolved in 100 g of water at 50°C.
What is solubility?Solubility is defined as the ability of a substance which is basically solute to form a solution with another substance. There is an extent to which a substance is soluble in a particular solvent. This is generally measured as the concentration of a solute present in a saturated solution.
The solubility mainly depends on the composition of solute and solvent ,its pH and presence of other dissolved substance. It is also dependent on temperature and pressure which is maintained.Concept of solubility is not valid for chemical reactions which are irreversible. The dependency of solubility on various factors is due to interactions between the particles, molecule or ions.
Learn more about solubility,here:
https://brainly.com/question/8591226
#SPJ1
Determine the total kilojoules in two tablespoons
Answers
The total kilojoules in two tablespoons is 836.8 kJ.
To determine the total kilojoules in two tablespoons of a substance, we need to know the specific substance and its energy content per tablespoon. Different substances have different energy values, so without this information, it is not possible to provide an accurate calculation.
The energy content of food or substances is typically measured in kilocalories (kcal) or kilojoules (kJ). 1 kilocalorie is equal to 4.184 kilojoules. The energy content of a substance is often listed on food labels or in nutritional databases.
For example, if we have the energy content of a substance as 100 kilocalories (kcal) per tablespoon, we can convert it to kilojoules by multiplying it by 4.184:
100 kcal * 4.184 kJ/kcal = 418.4 kJ
So, if we have two tablespoons of this substance, the total energy would be:
418.4 kJ/tablespoon * 2 tablespoons = 836.8 kJ
It's important to note that the energy content of a substance can vary depending on its composition, density, and other factors. Therefore, it is always recommended to refer to reliable sources such as food labels, nutritional databases, or consult a qualified professional to obtain accurate information regarding the energy content of specific substances.
For more such information on: kilojoules
https://brainly.com/question/29497478
#SPJ8
Radha takes some blue solution in a test tube and then puts some drops of lemon juice into it. She leaves the solution for an hour. After she comes back, she looks at the solution and concludes that a chemical change has happened in it. What might have occurred for her to conclude this? * (1 Point)
1.The solution evaporated from the test tube.
2.The drops of lemon juice formed a layer on the solution.
3.The solution changed colour from blue to red.
4.The drops of lemon juice increased the weight of the solution.
Answers
Answer:
The answer is 3 because If you dip blue litmus paper into lemon juice, it turns red. Because lemon juice is acidic, it makes blue litmus paper turn red. Because soapy water is alkaline, it turns red litmus paper blue
What volume would 0.435 moles of hydrogen gas, Hz, occupy at STP?
Answers
Answer:
will be 9.7 Liters
Explanation:
HF+H2o reacts to form ??
Answers
Answer:
Explanation:
HF + H2O reacts to form H3O+ (hydronium ion) and F- (fluoride ion).
The
is the wind system responsible for the movement of weather from west to east across most of the continental United States
A
polar easterlies
B
prevailing westerlies
C
trade winds
Answers
Answer:
Westerlies are prevailing winds that blow from the west at midlatitudes. They are fed by polar easterlies and winds from the high-pressure horse latitudes, which sandwich them on either side.Nov 15, C answer
When salt dissolves completely into water, which term is used to describe the water?
Answers
Answer:
A salt that is dissolved in water is called a solute
Once you have dissolved the salt - you have a solution
The water is called the solvent
Explanation:
We have that When salt dissolves completely into water the water is called a Solvent
it is important to not that once the salt compound comes into water it breaks down the molecule apart the Na on one hand and the Cl on the other hand
Once the NaCl is dissolved in to the H_2O it is known as a solute of salt
And when When salt dissolves completely into water the water is called a Solvent
Therefore
When salt dissolves completely into water the water is called a Solvent
For more information on this visit
https://brainly.com/question/1689737?referrer=searchResults
a) At 40 °C, what the saturated solubility level of KCl (aq)? (1 mark)
b) If 40 g of KCl is added to 100 g of water at 90 °C, would the solution be saturated? Explain. (2 marks)
c) Describe two ways in which saturation can be achieved if only 40 g of KCl is added to 100 g of water at 90 ° (3 marks)

Answers
a) The saturated solubility level of KCl (aq) at 40°C is 35.7 g/100 mL of water.
b) No, the solution would not be saturated at 90°C. At this temperature, the solubility of KCl in water is higher than at 40°C. Therefore, more KCl can dissolve in 100 g of water at 90°C than at 40°C. To determine if the solution is saturated, we need to compare the amount of KCl that actually dissolved in the water to the maximum amount of KCl that can dissolve in the water at that temperature. If the amount of KCl that dissolved is less than the maximum amount, then the solution is unsaturated. If the amount of KCl that dissolved is equal to the maximum amount, then the solution is saturated. If the amount of KCl that dissolved is greater than the maximum amount, then the solution is supersaturated.
c) Two ways in which saturation can be achieved if only 40 g of KCl is added to 100 g of water at 90°C are:
i) Heating the solution to a higher temperature: As the temperature of the solution is increased, the solubility of KCl in water also increases. Therefore, by heating the solution to a higher temperature than 90°C, more KCl can be dissolved in the water until the solution becomes saturated.
ii) Allowing the solution to cool slowly: If the solutionis heated to a temperature higher than 90°C and then allowed to cool slowly, the solubility of KCl in water decreases as the temperature decreases. This means that as the solution cools, KCl will begin to precipitate out of the solution until the solution becomes saturated. Alternatively, if the solution is left undisturbed at 90°C and allowed to cool slowly, KCl will begin to precipitate out of the solution as it reaches its saturation point.
Will give brainliest
In the following acid-base reaction
H2O is the
HCl(g) + H2O(l) →H30+(aq) + Cl-(aq)
В
acid
base
conjugate
acid

Answers
CONJUGATE ACID
the acid's conjugate base because it can theoretically reform the acid by aaccepting a proton.
I hope this is correct >.<
10-kg of R-134a at 300 kPa fills a rigid container whose volume is 14 L. Determine the temperature and total enthalpy in the container. The container is now heated until the pressure is 600 kPa. Determine the temperature and total enthalpy when the heating is completed.
Answers
Answer:
Temperature = 0.605°C
Total enthalpy at 300kpa = 545.2 kJ
Total enthalpy at 600kpa = 846.45 kJ.
Explanation:
Checking the table for 134a pressure table. It is given that the specific volume of saturated liquid and the specific volume of the saturated vapor of 280kpa is 0.0007699 m^3/kg and 0.072352 m^3/kg respectively.
Also, the specific volume of saturated liquid and the specific volume of the saturated vapor of 320kpa is 0.0007772 m^3/kg and 0.063604 m^3/kg respectively.
The first thing to do is to determine the value for the specific volume of saturated liquid.
At 300 kpa, the specific volume of saturated liquid,n is given below as;
300 - 280/ 320 - 280 = (n - 0.0007699)/ 0.0007772 - 0.0007699.
Therefore, n = specific volume of saturated liquid = 0.0007735 m^3/kg.
300 - 280/ 320 - 280 = n - 0.072352/ (0.063604 - 0.072352).
n = 0.0679 m^3/kg.
The second thing to do is to determine the value of the specific volume.
Specific volume = 14 × 10^-3/ 10 = 0.0014 m^3/kg.
Determine the enthalpy of the mixture,b(I). This is given below as;
300 - 280/ 320 - 280 = b(I) - 199.54/ (196.7 - 199.54).
b(I) = 198.125 kJ/Kg.
Hence, b = [ 300 - 280/ 320 - 280 = j - 50.18 / 55.16 - 50.18] + [ ( 0.0014 - 0.00077735) / 0.067978 - 0.00077735] × 198.125.
b = 54.517 KJ/Kg.
Total enthalpy = 10 × 54.517 = 545.17 kJ.
Temperature can be Determine as below;
300 - 280/ 320 - 280 = T + 1.25 / 2.46 - 1.25.
Temperature = 0.605°C.
Hence, at 600kpa, the total enthalpy = [81.51 + ( 0.0014 - 0.0008199/ 0.034295 - 0.0008199) × 180.90] × 10
Total enthalpy at 600kpa = 846.45 kJ.
What is the function of a lyase enzyme?
a. To assist the substrate by binding to the enzyme, enabling substrate to active site engagement
b. To facilitate a reaction of one substrate to form two products with the use of water
c. To facilitate a reaction of one substrate to form two products without the use of water
d. To tell fibs
Answers
Answer:
c. To facilitate a reaction of one substrate to form two products without the use of water
Explanation:
A lyase is an enzyme that catalyzes - accelerates the chemical reaction - in which a substrate is broken into two molecules. The reaction does not involve hydrolysis or oxidation, so the water molecule is not included in the chemical reaction. Thus, the enzyme facilitates the reaction in which a molecule (substrate) is decomposed into two molecules with the elimination of chemical bonds.
Aqueous aluminum bromide and chlorine gas react to form aqueous
aluminum
chloride and bromine gas
in a equation
Answers
Answer:
2AlBr₃\(_{aq}\) + 3Cl₂\(_{g}\) → 2AlCl₃\(_{aq}\) + 3Br₂\(_{g}\)
Explanation:
The word equation is:
Aqueous aluminum bromide and chlorine gas react to form aqueous aluminum chloride and bromine gas
Reactants:
aluminum bromide = AlBr₃
chlorine gas = Cl₂
Products:
aluminum chloride = AlCl₃
bromine gas = Br₂
So;
2AlBr₃\(_{aq}\) + 3Cl₂\(_{g}\) → 2AlCl₃\(_{aq}\) + 3Br₂\(_{g}\)