The total volume required to reach the endpoint of a titration required more than the 50 mL total volume of the buret. An initial volume of 49.17±0.04 mL was delivered, the buret was refilled, and an additional 1.56±0.04 mL was delivered before the endpoint was reached. The titration of a blank solution without the analyte required 0.60±0.04 mL . Calculate the endpoint volume corrected for the blank and its absolute uncertainty. Note: Significant figures are graded for this problem. To avoid rounding errors, do not round your answers until the very end of your calculations. volume: mL ± mL
Answers
Answer:
The answer is "\(\bold{50.42 \pm 0.08}\)".
Explanation:
Overall delivered volume \(= [(49.06 \pm 0.05) + (1.77 \pm 0.05)]\ mL\)
Its blank solution without any of the required analysis \(= (0.41 \pm 0.04)\ mL\)
Compute the volume of the endpoint as follows:
Formula:
\(\text{End point volume = Total Volume delivered - volume required}\)
\(= (49.06 \pm 0.05) + (1.77 \pm 0.05) - (0.41 \pm 0.04) \\\\= (49.06 + 1.77 - 0.41) \pm \ \ (absolute \ \ uncertainty)\)
therefore,
absolute uncertainty \(=\sqrt{(0.05)^2 + (0.05)^2 + (0.04)^2}\)
\(=\sqrt{0.0025 +0.0025 +0.0016} \\ \\=\sqrt{0.0066}\\\\=0.08124\\\)
The Endpoint volume \(= (49.06+1.77-0.41)\pm (0.08124)\)
\(= 50.42 \pm 0.08\)
Therefore, the volume of the endpoint adjusted for the blank is:
\(\bold { = 50.42 \pm 0.08}\)
Related Questions
What is the job of a scientist why do scientits need government funding
Answers
2. Scientist need government funding in order to maintain prestige.
Answer:
1. A scientist conducts and gathers research to gain knowledge in a particular area.
2. Many of the worlds problems include resources, energy, health, environment, climate, transportation, communication, etc. and will require solutions from science and engineering.
i need help with this, ive been trying to figure it out but i don’t understand. please number them 1-5 for the answers.

Answers
The solubility of the salts is affected by the temperature changes. 1. NaCl is least affected by temperature. 2. supersaturated. 3. 60 grams KBr. 4. Ethanol has both polar and non-polar groups. 5. Mixing and shaking.
A KBr solution with 90 gm solute in 100 grams of water at 50 degrees is classified as supersaturated. 60 grams of KBr are needed to make a saturated solution in 100 gm of water at 30 degrees.
Ethanol is a general solvent due to the presence of both the polar and the non-Polar groups. As a result, it is easier to dissolve both polar molecules and non-Polar molecules. The dissolving rate can be increased by mixing or shaking the solution. Also, the sugar dissolves faster in hot than cold tea.
To learn more about the solubility, refer to the link:
https://brainly.com/question/31493083
#SPJ1
The helium tank has a pressure of 650 torr at 25 degree celsius what will be the pressure if the temperature is tripled?
pa help po
Answers
The helium tank has a pressure of 650 torr at 25 degree Celsius and when the temperature is tripled, the pressure will be approximately 1945.71 torr
To find the new pressure when the temperature is tripled, we can use the ideal gas law, which states that the pressure of a gas is directly proportional to its temperature when the volume and the number of particles remain constant. The ideal gas law is given by the equation:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
First, we need to convert the initial temperature of 25 degrees Celsius to Kelvin. Adding 273.15 to the Celsius temperature gives us 298.15 K.
Let's assume that the volume, number of moles, and the gas constant remain constant.
If the temperature is tripled, the new temperature would be 3 times the initial temperature, which is 3 * 298.15 K = 894.45 K.
Now, we can set up a proportion to find the new pressure:
P1 / T1 = P2 / T2
Solving for P2 (the new pressure), we get:
P2 = (P1 * T2) / T1
Plugging in the values, we have:
P2 = (650 torr * 894.45 K) / 298.15 K
Calculating this expression, we find:
P2 ≈ 1945.71 torr
Therefore, when the temperature is tripled, the pressure will be approximately 1945.71 torr.
for more questions on pressure
https://brainly.com/question/24719118
#SPJ11
The _____ time it takes to completely _____ food, the _____ the food will deteriorate.
less -- unfreeze -- more
more -- hydrate -- less
less -- freeze -- less
Answers
The answer is C.less -- freeze -- less
solid zinc(ii) sulfide reacts with aqueous hydrobromic acid (hbr) to form aqueous zinc(ii) bromide and dihydrogen sulfide gas. express your answer as a chemical equation. identify all of the phases in your answer.
Answers
The balanced chemical equation for the reaction between solid zinc(II) sulfide and aqueous hydrobromic acid (HBr) to form aqueous zinc(II) bromide and dihydrogen sulfide gas is:
ZnS(s) + 2 HBr(aq) → ZnBr2(aq) + H2S(g)
In this equation, the phases of each substance are indicated in parentheses after the chemical formula.
The solid zinc(II) sulfide (ZnS) is indicated by the "(s)" phase, the aqueous hydrobromic acid (HBr) is indicated by the "(aq)" phase, the aqueous zinc(II) bromide (ZnBr2) is indicated by the "(aq)" phase, and the dihydrogen sulfide gas (H2S) is indicated by the "(g)" phase.
To know more about balanced chemical equation click on below link:
https://brainly.com/question/28294176#
#SPJ11
*75 POINTS NEED HELP FAST (Chemistry Gas Law Test Question)
If 1 mole of H₂ occupies 11.2 L at 0 °C, what volume will 4.00 moles of H₂ occupy at the same temperature and pressure?
Answers
The volume that 4.00 moles of H₂ will occupy at the same temperature and pressure is 44.8 L
Gas LawFrom the question, we are to determine the volume that 4.00 moles of H₂ will occupy
From the Ideal gas equation, we have that
PV = nRT
Where P is the pressure
V is the volume
n is the number of mole
R is the gas constant
and T is the temperature
Keeping the temperature and pressure are kept constant alongside the gas constant
Then,
V = kn
Then, we can write that
\(\frac{V }{n } = k\)
\(\frac{V_{1} }{n_{1} } = \frac{V_{2} }{n_{2} }\)
Where V₁ is the initial volume
n₁ is the initial number of mole
V₂ is the final volume
n₂ is the final number of mole
Then,
From the given information,
V₁ = 11.2 L
n₁ = 1 mole
n₂ = 4.00 moles
\(\frac{11.2}{1}=\frac{V_{2} }{4.00}\)
∴ V₂ = 4.00 × 11.2
V₂ = 44.8 L
Hence, the volume that 4.00 moles of H₂ will occupy at the same temperature and pressure is 44.8 L
Learn more on Gas Laws here: https://brainly.com/question/9185030
#SPJ1
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
Describe echolocation. Give an example of an animal that uses echolocation.
Answers
dolphin and whales use echolocation
Which change will most likely increase the rate of a chemical reaction? lowering the temperature of the reactants adding an inhibitor to the reactants increasing the concentration of one of the reactants
Answers
Answer:
By increasing the concentration of One of the reactants.
How much water needs to be added to a 8.6317 M NaCl solution to obtain a 1.0421 M solution, if you start with 4.7869 liters of solution?
Answers
The amount of water needed to be added to a 8.6317 M NaCl solution to obtain a 1.0421 M solution starting with 4.7869 liters of solution would be 34.8629 L.
DilutionThe problem here has to do with dilution. The dilution principle states that the number of moles of solute in a solution is constant before and after dilution.
In other words, the dilution principle can be mathematically expressed as:
\(m_1v_1\) = \(m_2v_2\).
Where \(m_1\) and \(m_2\) are molarities before and after dilution; \(v_1\) and \(v_2\) are volumes before and after dilution.
In this case: \(m_1\) = 8.6317 M, \(m_2\)= 1.0421 M, \(v_1\)= 4.7869 L. We are to determine v2.
\(v_2\) = \(m_1v_1\)/ \(m_2\)
= 8.6317x4.7869/1.0421
= 39.6498 L
If the final volume of the solution is 39.6498 L and the starting volume is 4.7869, then the amount of water needed can be calculated as:
39.6498 - 4.7869 = 34.8629 L
Thus, the amount of water that will be added to the original solution in order to arrive at the diluted solution is 34.8629 L.
More on dilutions can be found here: https://brainly.com/question/21323871
#SPJ1
Descriptions of enzyme mechanisms often contain the terms intermediate, transition state, and transition state analogue. The terms intermediate and transition state both describe chemical species along the reaction pathway, but there are important distinctions between the two. Transition state analogues are often used in enzyme mechanism studies. Match each phrase to the term that it describes.
a. Compound designed to mimic the structure of a transition state
b. Contains partially formed and partially broken bonds
d. Often serves as a potent enzyme inhibior
Answers
Solution :
The intermediate state and the transition state are used to describe the chemical species that is along the reaction pathway.
Therefore, the phrase of the term that it describes each of the terms, intermediate, transition state analogue and transition state are as follows :
a. Compound which is designed to mimic the structure of the transition state
-- transition state analogue
b. Containing partially formed as well as partially broken bonds.
-- transition state
d. Often serves as the potent enzyme inhibitor
-- transition state analogue
Which of the following is used to keep track of the heat flow inside a calorimeter?
Answers
Any time a liquid loses or receives energy, the temperature of the liquid changes.To calculate the amount pf energy change, the calorimeter monitors the weight of the fluid as well as the temperature change.
In a calorimeter, what thermometer is used?The 6000 series calorimeters' control systems serve as the foundation for the 6772 Calorimetric Thermometer, a high precision temperatures measuring device.It is a crucial component of both the 6755 Solution Calorimeter and the 6725 Semi-micro Calorimeter.
What equipment is utilized in calorimetry?The calorimeter is a piece of equipment used in calorimetry, a procedure for calculating heat capacity and measuring the temperature of chemical processes or other physical changes.Among the most popular kinds are differential scanning operating, thermal micro cathode rays, titration calorimeters, and accelerated rate calorimeters.
To know more about energy visit:
https://brainly.com/question/1932868
#SPJ1
What volume of hydrogen is required to react with 31 L of oxygen under the same conditions? 2 H2(g) + O2(g) → 2 H2O(g)
Answers
Answer:
62L
Explanation:
2 H2(g) + O2(g) → 2 H2O(g)
From the stoichiometry of the balanced equation;
2 mol of H2 reacts with 1 mol of O2.
From the law of combining volumes, equal moles of gases occupies equal volume.
This means;
2 L of H2 reacts with 1 L of O2
x L of hydrogen reacts with 31 L of O2
2 = 1
x = 31
Solving for x;
x = 62 L
Hello need help with question 9,10

Answers
1. 82554000 mg medicine should be given to 182 pound person
2. 0.036
Here in the 1st question given data is 8.70 kg of medicine per kg of body then, we have to calculate medicine should be given to 182 pound person = ?
Then we have to convert 182 pound into kg then,
1 pound = 0.454 kg
Then, 182 pound = 82.554 kg
Now we have to convert kg in mg then
1 kg = 10⁶ mg
Then, 82.554 kg = 82554000 mg
82554000 mg medicine should be given to 182 pound person
Here in the 2nd question given data is 36 joules then we have to calculate how many kilojoules = ?
So 1 joule = 0.001 kilojoule
Then 36 joules = 0.036 kilojoule
Here in 2nd question says that do not write answer in unit so, 36 joules = 0.036
Know more about conversion of units
https://brainly.com/question/28632397
#SPJ1
Find percent yield if 5.18 g of hydrogen gas and excess nitrogen gas were reacted to
produce 23.2 g of ammonia
Answers
The percent yield of ammonia in the reaction is 79.045%
The reaction of the formation of ammonia when hydrogen and nitrogen reacts is as follows:
N₂ + 3H₂ -------------> 2NH₃
It is given that 5.18g of Hydrogen gas and nitrogen gas reacted to produce 23.2g of Ammonia. To find the percent yield, the following formula is to be used:
Percent yield = Actual yield/ Theoretical yield x(100)
To calculate the actual yield of Ammonia, we get
Actual yield = (5.18g of H₂/1) x (1 mole of H₂ / 2g of H₂) x (2 moles of NH₃/ 3 moles of H₂) x (17g of NH₃/1 mole of NH₃)
= 5.18 x 0.5 x (34/3)
= 88.06/3
Actual yield = 29.35g
Therefore, the actual yield of ammonia is 29.35g
From this we can calculate the percent yield as follows:
Percent yield = 23.2/29.35 x(100)
Percent yield = 79.045%
Therefore the percent yield if 5.18 g of hydrogen gas and excess nitrogen gas were reacted to produce 23.2 g of ammonia is 79.045%
To know more about Haber's process, click below:
brainly.com/question/21867752
#SPJ9
PLEASE HELP...
Balance this nuclear reaction by supplying the missing nucleus. Replace each question mark with an appropriate integer or symbol.
Cf98249 + ? ⟶Db105260+410n
Answers
The balanced form of the nuclear equation is as follows; 249/98 Cf + 15/7 N⟶ 260/105 Db + 4(1/0) n.
What is a nuclear equation?A nuclear equation is process such as the fission of an atomic nucleus, or the fusion of one or more atomic nuclei and/or subatomic particles in which the number of protons and/or neutrons in a nucleus changes.
According to this question, Californium element is a reactant to produce dubnium and a neutron as products.
However, the law of conservation of mass must be fulfilled by ensuring the mass and atomic numbers of elements in reactant and product side are the same.
249/98 Cf + 15/7 N⟶ 260/105 Db + 4(1/0) n
Learn more about nuclear equation at: https://brainly.com/question/13315150
#SPJ1
What is the pH of a 50.0 mL solution of a 0.250 M HC2H3O2 solution after the
addition of 50 mL of 0.250 M NaOH to it? The Ka value for HC2H3O2 is 1.8 x 10-5
.
Answers
A 50.0 mL solution of 0.250 M HC₂H₃O₂ before and after adding 50 mL of 0.250 M NaOH has a pH of 2.87, the pH changes due to the formation of a buffer solution, which can be calculated using the Henderson-Hasselbalch equation.
How to calculate pH of the solution?This problem requires us to calculate the pH of a buffer solution after the addition of a strong base. A buffer solution is one that resists pH changes when modest amounts of acid or base are added. The buffer system in this case is the weak acid, acetic acid (HC₂H₃O₂) and its conjugate base, acetate ion (C₂H₃O₂-).
Before the addition of NaOH, we have a solution of 0.250 M HC₂H₃O₂, which we can assume to be completely dissociated in water:
HC₂H₃O₂ + H₂O ⇌ H₃O+ + C₂H₃O₂-
HC₂H₃O₂ has a Ka value of 1.8 x 10-5. We can use this Ka value to calculate the equilibrium concentration of H3O+ and C₂H₃O₂- in the solution.
First, we need to calculate the initial concentrations of HC₂H₃O₂ and C₂H₃O₂-.
moles of HC₂H₃O₂ = 0.250 M × 0.0500 L = 0.0125 mol
moles of C₂H₃O₂- = 0 mol (since there is no NaOH added yet)
Since HC₂H₃O₂ is a weak acid, it only partially dissociates in water. The equilibrium concentrations of H₃O+ and C₂H₃O₂- can be calculated using the Ka expression:
Ka = [H₃O+][C₂H₃O₂-]/[HC₂H₃O₂]
We can assume that the initial concentration of H3O+ is negligible compared to the amount that will be produced by the dissociation of HC₂H₃O₂, so we can simplify the expression to:
Ka = [H₃O+]²/[HC₂H₃O₂]
[H₃O+]² = Ka × [HC₂H₃O₂]
[H₃O+]² = 1.8 × 10⁻⁵ × 0.0125
[H₃O+] = 1.34 × 10⁻³ M
Now that we know the equilibrium concentration of H₃O+, we can use the pH formula to calculate the pH:
pH = -log[H₃O+]
pH = -log(1.34 × 10⁻³)
pH = 2.87
This is the pH of the buffer solution before the addition of NaOH.
Next, we add 50 mL of 0.250 M NaOH to the solution. NaOH is a strong base, so it completely dissociates in water to produce OH- ions:
NaOH → Na+ + OH-
The OH- ions will react with the acetate ions in the buffer solution to form water and acetate ions:
OH- + C₂H₃O₂- → H₂O + C₂H₃O₂-
This reaction will consume some of the acetate ions in the buffer solution, causing the equilibrium to shift to the left to produce more acetate ions.
To calculate the new concentrations of H₃O+ and C₂H₃O₂-, we need to use the Henderson-Hasselbalch equation:
pH = pKa + log([C₂H₃O₂-]/[HC₂H₃O₂])
where pKa = -log(Ka), [C₂H₃O₂-] is the equilibrium concentration of acetate ions, and [HC₂H₃O₂] is the equilibrium concentration of acetic acid.
To learn more about buffer solution, visit: https://brainly.com/question/8676275
#SPJ1
Sucrose (C₁₂H₂₂O₁₁) is combusted in air according to the following reaction:
C₁₂H₂₂O₁₁(s) + O₂(g) → CO₂(g) + H₂O(l)
How many grams of carbon dioxide would be produced by the complete combustion of 2.05 moles of sucrose in the presence of excess oxygen?
Answers
The mass of carbon dioxide that would be produced by the complete combustion of 2.05 moles of sucrose is 1,082.4 g.
Complete combustion of the sucroseThe complete combustion of sucrose in excess oxygen is given as follows;
C₁₂H₂₂O₁₁(s) + O₂(g) → CO₂(g) + H₂O(l)
balanced chemical equation is below;
C₁₂H₂₂O₁₁(s) + 12O₂(g) → 12CO₂(g) + 11H₂O(l)
from the reaction above;
1 mole of sucrose ----------> 12 mole of carbon dioxide
2.05 mole of sucrose ------> ?
= 2.05 x 12
= 24.6 moles
Mass of carbon dioxide1 mole of carbon dioxide = 44 g/mole
24.6 moles = ?
= 24.6 x 44
= 1,082.4 g
Thus, the mass of carbon dioxide that would be produced by the complete combustion of 2.05 moles of sucrose is 1,082.4 g.
Learn more about combustion reaction here: https://brainly.com/question/9425444
Consider the reaction: CO (g) + 2 H2 (g) ⇌ CH3OH (g) where the Kp is 2.26 x 10^4 at 25°C. Calculate ΔGrxn for the reaction at 25°C under standard conditions.
Answers
The standard Gibbs free energy change of the reaction at 25°C is -18,262 J/mol.
What is energy?Energy is the ability to do work. It exists in many forms, such as kinetic energy (energy of motion), potential energy (stored energy), thermal energy (heat), chemical energy (energy stored in the bonds of molecules), electrical energy (energy carried by electrons), and nuclear energy (energy produced in the nucleus of an atom).
The reaction given is a reversible reaction and the given Kp value is at 25°C. Therefore, we can use the equation ΔG°rxn = -RT ln Kp to calculate the standard Gibbs free energy change of the reaction.
We know that R = 8.314 J/mol K and T = 298 K (25°C)
Therefore, ΔG°rxn = - 8.314 J/mol K x 298 K x ln (2.26 x 10⁴)
ΔG°rxn = -18,262 J/mol
Therefore, the standard Gibbs free energy change of the reaction at 25°C is -18,262 J/mol.
To learn more about energy
https://brainly.com/question/29339318
#SPJ1
Please help!
Which atoms represent different isotopes of the same element? Picture included

Answers
"Option C" O¹⁵8 and O¹⁶8 atoms represent different isotopes of the same element.
Hope it helps...
Describe the buffer capacity of the acetic acid buffer solution in relation to the addition of both concentrated and dilute acids and bases. Reference the results in Data Tables 1,2,3, and 4 in your answer.
Answers
Answer:
The more concentrated acetic acid buffer has a better buffer capacity because requires more moles of acid or base to change the pH than a more diluted acetic acid buffer.
Explanation:
Buffer capacity is defined as the moles of an acid or base that are needed to change the pH of a buffer in 1 unit.
A more concentrated solution of acetic buffer contains more moles of the acid per liter of solution. A solution that contains more moles of the acetic ion or the acetic acid requires more moles of base or acid to change the pH, that means:
The more concentrated acetic acid buffer has a better buffer capacity because requires more moles of acid or base to change the pH than a more diluted acetic acid buffer.
2x²=8.pls help me i really need it
Answers
Explanation:
2x²=8
x²=8/2
x=√4
x=2
hope it helps.
Answer:
\(\huge \fbox \pink {A}\huge \fbox \green {n}\huge \fbox \blue {s}\huge \fbox \red {w}\huge \fbox \purple {e}\huge \fbox \orange {r}\)
\( {2x}^{2} = 8 \\ {x}^{2} = \frac{8}{2} \\ {x}^{2} = 4 \\ x = \sqrt{4} \\ x = 2\)
ʰᵒᵖᵉ ⁱᵗ ʰᵉˡᵖˢ
\( \huge\purple{ \mid{ \underline{ \overline{ \tt ꧁❣ ʀᴀɪɴʙᴏᴡˢᵃˡᵗ2²2² ࿐ }} \mid}}\)
O
Question 2 of 10
Which of the following values best classifies a bond between 2 atoms as
being covalent?
A. An electronegativity difference of greater than 2.7 between the
atoms
B. An electronegativity difference of greater than 1.7 between the
atoms
C. An electronegativity difference of less than 1.7 between the atoms
D. An electronegativity difference of less than 2.7 between the atoms
← PREVIOUS
SUBMIT
Answers
A bond between 2 atoms as being covalent an electronegativity difference of greater than 1.7 between the atoms.
What is bond?Bond is a debt instrument, which is issued by governments and corporations. These bonds are used to raise capital for various projects and investments. Bonds are a form of loan and investors are usually paid a fixed interest rate for lending their money. Bonds are typically issued for a fixed period of time, usually for a period of 5-30 years, and have a fixed maturity date. Investors receive interest payments at regular intervals and receive the principal amount of the bond at the end of the period.
To learn more about bond
https://brainly.com/question/29794367
#SPJ1
need it for resources(duh)

Answers
Answer: Trail 2
Explanation: Because it has a pressure of 1 atm
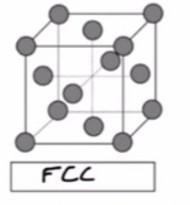
BaCl2 crystallizes such that the Ba2+ ions are in a face-centered cubic arrangement and the Cl- ions are in the holes of the lattice (fluorite structure). How many Cl- ions are present in one unit cell of this crystal?
Answers
BaCl2 crystallizes such that the Ba2+ ions are in a face-centered cubic arrangement and the Cl- ions are in the holes of the lattice (fluorite structure). The numbers of Cl- ions present in one unit cell of this crystal is 8.
The Cubic system arrangement is one in which the unit cell is shaped like a cube.
A face centered cubic arrangements (FCC) is an atom arrangement located in nature. FCC structural unit cell is made up of atoms organized in a cube with fraction of an atom in each corner and six additional whole atoms in the middle of the cube face.
From the information given;
Ba2+ is found in the FCC arrangementsThe total number of Ba2+ atom present within the unit cell can be computed as:
\( \mathbf{(6 \ face \: \: sharing \: atom)( \dfrac{1}{2}) \: + (8 \: edges)( \dfrac{1}{8} \: corner \: sharing \: atoms)}\)
\( \mathbf{ = 4 \: atoms}\)
From the formula for BaCl2; since there is twice number of Cl- present as that of Ba2+;
Then, the number of Cl- present per unit cell is:= 4 × 2= 8Therefore, we can conclude that the numbers of Cl- ions present in one unit cell of this crystal is 8.
Learn more about cubic arrangements here:
https://brainly.com/question/21826251?referrer=searchResults
An atomic cation with a charge of +1 has the following electron configuration:
1s 2s 2p 3s 3p 3d ¹4s¹
What is the chemical symbol for the ion?
How many electrons does the ion have?
How many 3p electrons are in the ion?
Answers
A) The chemical symbol for the ion is Fe+
B) It has 20 electrons in total, and there are 6 3p electrons in the ion.
C) There are 6 electrons present in the 3p orbital.
The atomic cation with the given electron configuration is represented by the chemical symbol Fe+.
To determine the number of electrons in the ion, we need to count the electrons present in the electron configuration. In the given configuration, we can see that the 1s orbital has 2 electrons, the 2s orbital has 2 electrons, the 2p orbital has 6 electrons, the 3s orbital has 2 electrons, the 3p orbital has 6 electrons, the 3d orbital has 1 electron, and the 4s orbital has 1 electron. Adding up these numbers, we have:
2 + 2 + 6 + 2 + 6 + 1 + 1 = 20
Therefore, the ion has 20 electrons.
To determine the number of 3p electrons in the ion, we look at the 3p orbital. In this case, there are 6 electrons present in the 3p orbital.
In summary, the chemical symbol for the ion is Fe+, it has 20 electrons in total, and there are 6 3p electrons in the ion.
For more question on electrons
https://brainly.com/question/26084288
#SPJ8
What is the oxidation number of chlorine in KClO3?
Group of answer choices
A. +5
B. +1
C. -5
D. -1
Answers
The oxidation number of chlorine in KClO3 is +5 (option A).
How to calculate oxidation number?The oxidation number of an element is the hypothetical charge of an atom within a molecule.
The oxidation number of an element like chlorine in a compound like Pottasium chlorate can be calculated as follows:
The oxidation number of the elements in KClO3 is as follows:
K = +1Cl = xO = -21 + x - 2(3) = 0
x - 5 = 0
x = +5
Therefore, the oxidation number of chlorine in KClO3 is +5.
Learn more about oxidation number at: https://brainly.com/question/15167411
#SPJ1
What is the pH of a solution the hydroide concentration of 1 X 10.5?
Answers
Answer:
Explanation:
Therefore, the pH of the HCl solution is 5.
3.124. X-ray Absorption Spectroscopy Electrons in multielectron
atoms absorb X-rays at characteristic energies, leading to
ionization. The characteristic energies for each element allow
scientists to identify the element. For magnesium, X-rays
with λ = 952 pm are required to selectively eject an electron
A
from the n = 1 energy level, and X-rays with λ = 197 nm
remove an electron from the n = 2 energy level.
a. Calculate the frequency and energy of those X-rays.
b. Why are the wavelengths and energies different for the
two electrons?
c. In terms of Bohr's values of n, what transitions do those
represent?
Answers
Answer:
ddddddddddddedewxdedww
Explanation:
ddddddddddddddddddddddd
When the seed is fertilized, the sprout turns into an adult before a seedling?