Answers
Hydrogen was is collected over water with a total pressure of 725.0 mmHg at 18.5 °C. The partial pressure of hydrogen gas is 708.9 mmHg.
What is collecting gas over water?When a gas is produced in a chemical reaction, it can be collected by water displacement.
Because the gas is collected over water, it is not pure but is mixed with vapor from the evaporation of the water.
What does Dalton's law state?Dalton's law state that the total pressure of a gaseous mixture is equal to the sum of the partial pressures of the gases.
The total pressure of gas collected over water is 725.0 mmHg and the temperature is 18.5 °C.
At that temperature, the vapor pressure of water is 16.13 mmHg.
P = pGas + pH₂O
pGas = P - pH₂O = 725.0 mmHg - 16.13 mmHg = 708.9 mmHg
Hydrogen was is collected over water with a total pressure of 725.0 mmHg at 18.5 °C. The partial pressure of hydrogen gas is 708.9 mmHg.
Learn more about collecting gases over water here: https://brainly.com/question/13323291
Related Questions
A piece of lumber is 7.60cm long. What is it’s length in millimetres and in inches?
Answers
Explanation:
Since 10mm is 1cm
Therefore 7.60cm is 760mm
Since 1 inch is 2.54cm
Therefore 7.60cm is 3.10 inches
A student planned to make copper sulfate crystals from excess copper oxide and dilute sulfuric acid.
The equation for the reaction is:
CuO(s) + H,SO (aq) -, CuSO (aq) + H20(1)
This is the method used.
1. Add 25 cm° of dilute sulfuric acid to a conical flask.
2. Gently warm the dilute sulfuric acid.
3. Add excess copper oxide to the dilute sulfuric acid.
4. Stir the mixture.
5. Heat to evaporate all the water from the mixture.
Suggest two improvements to the method
Explain why each improvement is needed.
A student plans a method to prepare pure crystals of copper sulfate.
The student's method is:
1. Add one spatula of calcium carbonate to dilute hydrochloric acid in a beaker.
2. When the fizzing stops, heat the solution with a Bunsen burner until all the liquid is gone.
The method contains several errors and does not produce copper sulfate crystals.
Explain the improvements the student should make to the method so that pure crystals of copper sulfate are produced.

Answers
The student's method for preparing pure crystals of copper sulfate contains errors and does not produce the desired outcome.
Use copper oxide instead of calcium carbonate: The student should add copper oxide (CuO) to the hydrochloric acid instead of calcium carbonate. Copper oxide reacts with hydrochloric acid to form copper chloride, which can then be converted to copper sulfate through a subsequent reaction with sulfuric acid.
Add sulfuric acid to the copper chloride solution: After the copper chloride solution is formed, the student should add sulfuric acid to it. This reaction between copper chloride and sulfuric acid will yield copper sulfate and hydrochloric acid. The student should ensure that the correct stoichiometric ratio is maintained to maximize the yield of copper sulfate crystals.
Crystal formation: The student should allow the solution to cool slowly after the reaction with sulfuric acid. This promotes the formation of larger, well-defined copper sulfate crystals.
Filtration and drying: Once the crystals have formed, the student should filter the solution to separate the solid crystals from the remaining liquid. The filtered crystals should then be thoroughly dried to remove any remaining water, resulting in pure copper sulfate crystals.
By following these improvements, the student can obtain pure crystals of copper sulfate.
For more such questions on copper sulfate visit:
https://brainly.com/question/17439051
#SPJ8
How many grams of oxygen gas would be needed to react with 10. grams of hydrogen gas?
Answers
10. grams of hydrogen gas is equal to 5 moles of hydrogen gas. In order to react with this amount of hydrogen gas, 5 moles of oxygen gas is required, which is equal to 160 grams of oxygen gas.
What is hydrogen gas?Hydrogen gas is a colorless, odorless, tasteless, and flammable gas. It is the lightest and most abundant element in the universe, making up around 75% of the universe's elemental mass. Hydrogen can exist in several states, including molecular hydrogen (H2), atomic hydrogen (H), and ionized hydrogen (H+). In its molecular form, hydrogen is a non-toxic and non-polluting gas that can be used to produce electricity, fuel, and other chemicals.
In order to answer this question, you must first understand the stoichiometry of the reaction between hydrogen and oxygen.
The balanced equation for this reaction is:
2H2 + O2 → 2H2O
This means that for every two moles of hydrogen gas, one mole of oxygen gas is required. The molar mass of hydrogen gas is 2.016 g/mol, and the molar mass of oxygen gas is 32.00 g/mol.
To learn more about hydrogen gas
https://brainly.com/question/19813237
#SPJ1
The _______________________________________ is often referred to as the powerhouse of the cell
Answers
Answer:
Mitochondria
Explanation:
Answer:
Mitochondria
Explanation:
Mitochondria are tiny organelles inside cells that are involved in releasing energy from food. This process is known as cellular respiration. It is for this reason that mitochondria are often referred to as the powerhouses of the cell.
A solution of ammonia and water contains 4.00×1025 water molecules and 9.00×1024 ammonia molecules. How many total hydrogen atoms are in this solution?
Answers
According to the information provided, there are 8.601024 8.60 10 -24 ammonia molecules in a solution of water and ammonia (NH3 N H 3). The total number of hydrogen atoms inside the solution is 1.06 1026.
How are hydrogen atoms of water calculated?Water has the chemical formula H2O, which stands for two moles of hydrogen and one mole for oxygen. Consequently, there are two moles of hydrogen in a single water molecule. 18 g if water will have 1 x 12.046 1023 = 12.046 1023 hydrogen atoms.
How many water molecules are there, exactly?According to Avogadro's number, there's many 6.022 x 1023 water molecules in a mole of water. After determining that a drop of water has 0.002775 moles, we can now calculate how so many molecules are present in it: molecules inside a water drop Equal (6.022 x 1023 particles) x 0.002275 moles.
To know more about ammonia visit:
https://brainly.com/question/15409518
#SPJ1
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
I NEED HELP!!OMG OMG OMG

Answers
Winter is purple, spring is blue, summer is orange, fall is green.
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
Calculate the frequency of light having wavelength of 456 nm.
Answers
Answer:
Calculate the frequency of light having wavelength of 456 nm.
1.22*10^8nm
Explanation:
In the given question, \(6.58 \times 10^{14}\) Hz is the frequency of the light with a wavelength of 456 nm.
Frequency is a measure of the number of cycles of a periodic wave that occur per unit of time.
The frequency of light can be calculated using the following equation:
frequency = speed of light / wavelength
where the speed of light is a constant equal to \(\rm 3 \times 10^8 \ m/s\).
To use this equation, we need to convert the wavelength from nanometers to meters:
456 nm = \(\rm 456 \times 10^{-9}\ m\)
Now, we can substitute the values into the equation:
frequency = \(\rm 3 \times 10^8 \ m/s\) / \(\rm 456 \times 10^{-9}\ m\)
= \(6.58 \times 10^{14}\) Hz
Therefore, the frequency of light with a wavelength of 456 nm is \(6.58 \times 10^{14}\) Hz.
Learn more about frequency here:
https://brainly.com/question/29739263`
#SPJ4
How many grams of F2 gas are there in a 5.00-L cylinder at 4.00 × 10^3 mm Hg and 23°C?
Answers
Answer:
41.17g
Explanation:
We are given the following parameters for Flourine gas(F2).
Volume = 5.00L
Pressure = 4.00× 10³mmHG
Temperature =23°c
The formula we would be applying is Ideal gas law
PV = nRT
Step 1
We find the number of moles of Flourine gas present.
T = 23°C
Converting to Kelvin
= °C + 273k
= 23°C + 273k
= 296k
V = Volume = 5.00L
R = 0.08206L.atm/mol.K
P = Pressure (in atm)
In the question, the pressure is given as 4.00 × 10³mmHg
Converting to atm(atmosphere)
1 mmHg = 0.00131579atm
4.00 × 10³ =
Cross Multiply
4.00 × 10³ × 0.00131579atm
= 5.263159 atm
The formula for number of moles =
n = PV/RT
n = 5.263159 atm × 5.00L/0.08206L.atm/mol.K × 296K
n = 1.0834112811moles
Step 2
We calculate the mass of Flourine gas
The molar mass of Flourine gas =
F2 = 19 × 2
= 38 g/mol
Mass of Flourine gas = Molar mass of Flourine gas × No of moles
Mass = 38g/mol × 1.0834112811moles
41.169628682grams
Approximately = 41.17 grams.
The mass of F₂ in the 5 L cylinder at 4×10³ mmHg and 23 °C is 41.154 g
We'll begin by calculating the number of mole of F₂ in the cylinder. This can be obtained as follow:Volume (V) = 5 L
Pressure (P) = 4×10³ mmHg
Temperature (T) = 23 °C = 23 + 273 = 296 K
Gas constant (R) = 62.364 mmHg.L/Kmol
Number of mole (n) =?PV = nRT
4×10³ × 5 = n × 62.364 × 296
20000 = n × 18459.744
Divide both side by 18459.744
n = 20000 / 18459.744
n = 1.083 moleFinally, we shall determine the mass of F₂ in the cylinder.Mole of F₂ = 1.083 mole
Molar mass of F₂ = 2 × 19 = 38 g/mol
Mass of F₂ =?Mass = mole × molar mass
Mass of F₂ = 1.083 × 48
Mass of F₂ = 41.154 gTherefore, the mass of F₂ in the cylinder is 41.154 g
Learn more: https://brainly.com/question/15214204
The density of aluminium is 2.7 g/cm3. Find the mass in grams of a bar of aluminum measuring 1.7 cm by 3.0 cm by 12.9 cm.
Answers
Answer: 177.23 g.
Explanation:
the volume of the aluminum bar is
1.7 cm x 3.0 cm x 12.9 cm
= 65.61 cm^3
2.7 g/cm^3 x 65.61 cm^3
177.23g
Problem #4: Interpret reactions in terms of representative particles, then write balanced chemical
equations and compare with your results. Determine limiting and excess reagent and the amount of
unreacted excess reactant.
a) 3 atoms of carbon combine with 4 molecules of hydrogen to produce methane (CH4)
b) 7 molecules of hydrogen and 2 molecules of nitrogen gases react to produce ammonia
c) 4 molecules of hydrogen and 5 molecules of chlorine react.
Answers
Answer:
a) The balanced chemical equation for the reaction is:
3C (s) + 4H2 (g) → CH4 (g)
In this reaction, 3 carbon atoms combine with 4 molecules of hydrogen to produce one molecule of methane. The representative particles in this reaction are atoms of carbon and molecules of hydrogen and methane.
b) The balanced chemical equation for the reaction is:
2N2 (g) + 7H2 (g) → 2NH3 (g)
In this reaction, 7 molecules of hydrogen react with 2 molecules of nitrogen gas to produce 2 molecules of ammonia. The representative particles in this reaction are molecules of hydrogen, nitrogen, and ammonia.
c) The balanced chemical equation for the reaction is:
H2 (g) + Cl2 (g) → 2HCl (g)
In this reaction, 4 molecules of hydrogen react with 5 molecules of chlorine gas to produce 10 molecules of hydrogen chloride. The representative particles in this reaction are molecules of hydrogen, chlorine, and hydrogen chloride.
To determine the limiting and excess reagents and the amount of unreacted excess reactant, we need to know the initial amounts of each reactant. Without this information, we cannot determine the limiting and excess reagents or the amount of unreacted excess reactant.
list three statements for transverse waves
Answers
•Transverse waves are a form of traveling energy
•Transverse waves have oscillations perpendicular to the direction of energy transfer
•Transverse waves have particles that move about their fixed position
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
Zeros between nonzero digits are significant
Answers
Answer:
Explanation:
If a zero is found between significant digits, it is significant
What type of temperatures and moisture content does Marine time Tropical air have?
Group of answer choices
Cold Temps/ Moist Air
Warm Temps/ Dry Air
Cold Temps/ Dry Air
Warm Temps/ Moist Air
Answers
Answer:
D: Warm Temps/ Moist Air
Explanation:
Tropical places usulay have warm mosit air
Maritime Tropical air is associated with warm temperatures and moist air.
Maritime Tropical (mT) air is a type of air mass that forms over warm tropical or subtropical ocean regions. It acquires its characteristics from the ocean's influence, particularly its warm temperatures and high moisture content.
Warm Temperatures: Maritime Tropical air masses originate from regions near the equator or subtropics, where solar radiation is intense and consistently high. As a result, these air masses are typically warm, with temperatures ranging from mild to hot.
The term "Maritime" refers to air masses that have originated from over the ocean, which generally have higher moisture content due to evaporation from the ocean surface. The term "Tropical" indicates that the air mass originates from or near tropical regions, where warm temperatures are prevalent.
Therefore, the correct answer is: Warm Temps/ Moist Air.
Learn more about Maritime Tropical from the link given below.
https://brainly.com/question/32262099
#SPJ2
Write down the chemical equation of the reaction that we performed in this lab
Answers
This is an riddle.
I’m lighter than what I am made of and more of me is hidden than is seen. What am I?
Whoever answers correctly gets brainly.
Answers
Answer: The answer is Iceberg
Answer:
Is it an iceberg?
Explanation:
it floats in water
and you can only see the tip of an iceberg
Hope this helps! :)
Have a great weekend!!
In the context of small molecules with similar molar masses, arrange the intermolecular forces by strength.
a. London dispersion forces
b. hydrogen bonding
c. dipole-dipole interactions
Answers
Answer:
Hydrogen bonding - London dispersion forces - dipole-dipole interactions
Strongest ----> Weakest
Hola alguien me puede ayuda esto no se como

Answers
K=c +273
Ósea que en vez de c vas a poner el número
Para de Kevin a Celsius
C=k - 273
12. Photosynthesis builds sugars out of small molecules, making it an
Answers
Answer:
Condensation reaction/ direct synthesis reaction
Explanation:
Combines simple molecules to form complex molecules producing water
How many grams are contained in the quantity 0.34 mol Ca
Answers
Answer:
13.6272 Grams
Explanation:
Moles of Calcium × atomic weight = Grams
0.34 × 40.08 = 13.6272
0.34 Moles of Calcium = 13.6272 Grams
KF + 02
Balance the equation
Answers
Explanation:
this equation is balanced
if you look at it carefully
k=1
f=1
o=2
we do not have any opposing element
Answer:
this equation is balanced
if you look at it carefully
k=1
f=1
o=2
we do not have any opposing element
19. A girl is ice skating and has 35 kgm/s of momentum. After she bumps into a
friend, she has 25 kgm/s of momentum. How much did she give her friend?
Answers
Answer:
15 kgm/s of momentum
Explanation:
How many significant figures is this and 40.5184 rounded
Answers
Answer:
forty point five one eight four
Explanation:
40.5184
Sig Figs
6
40.5184
Decimals
4
40.5184
Scientific Notation
4.05184 × 101
E-Notation
4.05184e+1
Words
forty point five one eight four
—————
—————
—————
\/

What element is in 3rd period and the 1st group?
Answers
Answer:
That is sodium
Explanation:
Sodium is found there
Use the periodic table to predict which ion will form from each main-group element.
Na:
F:
N:
I:
Sr:
Answers
The ions that are formed by the ions is;
Na^+F^-N^3-I^-Sr^2+What is an ion?The term ion refers to a specie that is obtained by the loss or gain of electrons. A positive ion is formed by the loss of electrons while a negative ion is formed by gain of electrons.
A positive ions has more protons than electrons while a negative ion has more electrons than protons. There is only one major ion that could be formed by main group elements.
The ions that are formed by the ions is;
Na^+F^-N^3-I^-Sr^2+Learn more about ions:https://brainly.com/question/14982375
#SPJ1
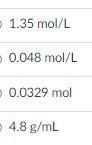
What is the molarity of 1.20 g of HCl in 25.0 mL of water?
Answers
Answer:
The molarity of the solution is 1.23M.
Explanation:
1st) To calculate the molarity, it is necessary to have the moles of the solute per liter (or 1000mL) of solution. So, we have to convert 1.20g of HCl to moles, using the molar mass of HCl (36.5g/mol):
\(\begin{gathered} 36.5g-1molHCl \\ 1.20g-x=\frac{1.20g*1molHCl}{36.5g} \\ x=0.032molHCl \end{gathered}\)2nd) Now we know that 0.032 moles of HCl are contained in 25.0mL of water.
HCl is the solute and water is the solvent, so with the density of HCl (1.3g/mL) we have to calculate the mL of HCl:
\(\begin{gathered} Density=\frac{mass}{volume} \\ 1.3\frac{g}{mL}=\frac{1.20g}{volume} \\ volume=\frac{1.20g}{1.3\frac{g}{mL}} \\ volume=0.923mL \end{gathered}\)Now, adding the solute plus the solvent, we get the volume of the solution:
\(\begin{gathered} Volume_{solution}=Volume_{solute}+Volume_{solvent} \\ Volume_{solution}=0.923mL+25.0mL \\ Volume_{solution}=25.923mL \end{gathered}\)3rd) Finally, we can calculate the molarity of the solution with a mathematical rule of three:
\(\begin{gathered} 25.923\text{mL solution-0.032moles HCl} \\ 1000\text{ mL solution - x=}\frac{1000\text{ mL solution}*0.032molesHCl}{25.923\text{mL solution}} \\ x=1.23\text{moles HCl} \end{gathered}\)So, the molarity of the solution is 1.23M.
Make two molecules of water without any leftovers, what is the result?
Answers
To create molecules, atoms combine. Two hydrogen (H) atoms and one oxygen (O) atom make up a water molecule's three atoms. H2O is a common abbreviation for water because of this.
EXAMPLES OF MOLECULES AND WHAT A MOLECULE IS:The smallest unit of a material that keeps its content and characteristics is a molecule, which is made up of two or more atoms linked together by chemical bonds. The building blocks of chemistry are molecules.
What exactly are fundamental molecules?The phrase "the molecules of life" frequently refers to these four categories of molecules. Proteins, carbohydrates, lipids, and nucleic acids make up the four basic building blocks of life. Every single life on Earth depends on one or more of the four categories. These four molecules are necessary for a cell and an organism to exist.
To know more about molecules visit:
https://brainly.com/question/19922822
#SPJ1
Predict which of the following reactions has a positive change in entropy.
l. 2N2(g) + O2(g) → 2N2O(g)
II. CaCO3(s) → CaO(s) + CO2(g)
III. Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Answers
Several factors can dictate entropy in an equation.
These include:
1. Phase changes
⇒ When a solid turns to a liquid, the entropy increases as the particles have more freedom to move around and thus have a greater ability for 'disorder'. Same goes for a liquid turning to a gas. In a gas, the intermolecular forces are much weaker than that of a solid or liquid, allowing the particles more freedom.
So, going from a solid to liquid to gas increases entropy, and going the other way, from gas to liquid to solid, decreases entropy.
Example:
H₂O(l) -> H₂O(g)
This will have a positive entropy change, as the water molecules are becoming gaseous and thus have more freedom.
2. Dissolution
⇒ Similarly, breaking up particles of a solute when dissolving in a solvent will increase entropy as the particles are no longer bound together.
So, dissolving a solute will increase entropy.
Example:
NaCl(s) -> NaCl(aq)
This will have a positive entropy change, as the NaCl particles are more free after being separated.
3. Number of products and reactants
⇒ Generally, if you have more moles of products than reactants, if they are the same phase then entropy will increase. Note this is not necessarily true if you form a gas from two non-gas reactants, as the gas will still have more entropy.
4. Temperature
⇒ Increasing temperature will increase entropy as the particles have more kinetic energy and are then moving faster.
-------------------------------------------
l. 2N2(g) + O2(g) → 2N2O(g)
3 moles of gas are forming 2 moles of gas. The phase of products and reactants are the same, so since we have less moles of product than reactant, entropy will be negative.
II. CaCO3(s) → CaO(s) + CO2(g)
1 mole of solid is forming 1 mole of solid and 1 mole of gas. There is a phase change from solid to gas, and there are more moles of product than reactant, entropy will be positive.
III. Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
While 3 moles of reactant are forming only 2 moles product, we are forming a gas from non-gaseous reactants, so entropy will be positive regardless.