The temperature of sulfur dioxide is changed, causing a change in volume from 20. 923 L to 29. 508 L. If the new temperature is 260. 93 K,
what was its original temperature?
Your answer must include the following:
• The name of the law that applies to this problem
• The equation that you are going to use expressed in variables
• The answer with correct units
Answers
The law that applies to this problem is Charles's Law.
The equation for Charles's Law is \(\frac{V_{1} }{T_{1} }\) = \(\frac{V_{2} }{T_{2} }\)
The original temperature of sulfur dioxide was 185.12 K.
The law that applies to this problem is Charles's Law, which states that at constant pressure, the volume of a fixed amount of gas is directly proportional to its temperature in kelvin.
The equation for Charles's Law is \(\frac{V_{1} }{T_{1} }\) = \(\frac{V_{2} }{T_{2} }\), where \(V_{1}\) is the initial volume, \(T_{1}\) is the initial temperature, \(V_{2}\) is the final volume, and \(T_{2}\) is the final temperature.
Using the given values, we can plug them into the equation and solve for the initial temperature:
\(\frac{V_{1} }{T_{1} }\) = \(\frac{V_{2} }{T_{2} }\)
20.923/\(T_{1}\) = 29.508/260.93
Multiplying both sides by \(T_{1}\) and dividing by 29.508, we get:
\(T_{1}\) = (20.923/29.508) x 260.93 = 185.02 K
Therefore, the original temperature of sulfur dioxide was 185.12 K.
The answer with correct units is 185.12 K.
To know more about Charles's Law visit:
https://brainly.com/question/16927784
#SPJ11
Related Questions
do all-stars mak energy through fusions
Answers
what is the percent yield for a reaction where the theoretical yield of the product was 45.9 g while the actual yield obtained in the lab for this product was 39.6 g?
Answers
a sealed flask at 298 k is half full of water. which is a correct statement after the flask has been heated to 308 k and the equilibrium has re-established?
Answers
When the flask has been heated to 308 K and the equilibrium has been re-established, the volume of water in the flask will have changed. It is known that the density of water decreases as the temperature increases. This implies that, when the flask is heated, the volume of water will expand.
As a result, the pressure in the flask will rise due to the water vapor that has accumulated as a result of the increased temperature.
After the temperature has been increased and the equilibrium re-established, the correct statement will be that the water level has increased in the flask, and the pressure has also increased. This implies that the water molecules in the flask have expanded as a result of the increased temperature.
In addition, the increased volume of water molecules increases the number of collisions of water molecules with the flask walls.
Therefore, the water molecules in the flask absorb some energy from the heat source and then transfer it to the flask walls in the form of collisions. As a result of these increased collisions, the pressure in the flask increases. This is because the number of collisions between water molecules and the walls of the flask has increased as a result of the increased temperature.
In conclusion, when the sealed flask is heated to 308 K, the volume of water in the flask increases, and the pressure inside the flask also increases. This is due to the increased number of collisions between water molecules and the flask walls, which are caused by the increased volume of water molecules due to the increase in temperature.
To know more about equilibrium here
https://brainly.com/question/30694482
#SPJ11
Here is the actual question from before, I forgot to put the question in 0-0

Answers
Answer:
Evaluate \dfrac {9}m+4
m
9
+4start fraction, 9, divided by, m, end fraction, plus, 4 when m=3m=3m, equals, 3.
Explanation:
The Sun keeps all of the planets orbiting it because _____.
A. he Law of Ellipses states that the planets orbit the Sun in circular patterns
b. the Law of Universal Gravitation states that an increase in mass causes an increase in gravitational force
C. the Law of Harmonies states that the motion of the planets will not stop unless an outside force is applied
D. the Law of Equal Areas states that planets sweep equal areas in equal periods of time around the Sun
Answers
Answer:
a. because of how the earth circulates around the sun.
Which solution contains the biggest amount of chloride ions?
I don’t understand why it’s B not D

Answers
Answer:
the quantity of solution to moles for d is significantly lower.
Explanation:
the larger the amount of liquid, the less amount of ions to dissociate hence having a lesser amount of chloride ions.
compared to b which is less liquid and a higher concentration
Solution B (MgCl₂) has a molarity of 0.20 mol/dm³ and a volume of 60 cm³.
Option (B) is correct.
First, we need to convert the volume from cubic centimeters (cm³) to cubic decimeters (dm³) because the molarity is given in moles per cubic decimeter (mol/dm³). There are 1000 cm³ in 1 dm³, so:
Volume = 60 cm³ × (1 dm³ / 1000 cm³) = 0.060 dm³
Now we can calculate the moles of chloride ions in solution B:
Moles = Molarity × Volume = 0.20 mol/dm³ × 0.060 dm³ = 0.012 moles
None of the other options have both a high molarity and a high volume to yield a higher amount of chloride ions. Option A has a higher molarity but a smaller volume, option C has a higher volume but a smaller molarity, and option D doesn't have a high molarity or volume for chloride ions.
Therefore, among the given options, solution B (MgCl₂) contains the largest amount of chloride ions, which is 0.012 moles.
To learn more about molarity here
https://brainly.com/question/32787122
#SPJ3
The complete question is:
Which solution contains the biggest amount, in mol, of chloride?
A. 20 cm³ of 0.50 mol dm⁻³NH,CL
B. 60 cm³ of 0.20 mol dm⁻³ MgCl₂
C. 70 cm³ of 0 30 mol dm⁻³ NaCl
D. 100 cm³ of 0.30 mol dm⁻³ CICH₂ COOH
Consider the titration of 30. 0 mL of 0. 050 M NH3 with 0. 025 M. HCl. Calculate the PH after the following volumes of titrant have been added 0 ml 20 mL 59. 1 mL 60. 0 mL 71. 4 mL 73. 4 mL
Answers
The pH after the following volumes of titrant have been added 0 ml 20 mL 59. 1 mL 60. 0 mL 71. 4 mL 73. 4 mL are 11.89, 11.89, 8.45, 8.45, 7.98, 8.95 respectively.
The reaction between NH3 and HCl can be represented by the following equation: NH3 + HCl → NH4+ + Cl-
To calculate the pH after different volumes of titrant have been added, we need to determine the amount of titrant that has reacted with the analyte and the resulting concentration of the products.
A. 0 mL of titrant (initial state)
At the start, there is no titrant added to the analyte, so the concentration of NH3 is 0.050 M. NH3 is a weak base, so we can use the Kb expression to calculate the concentration of OH-:
\(Kb = [NH4+][OH-] / [NH3]\)
\(1.8 * 10^{-5} = x^2 / (0.050 - x)\)
initial concentration of NH3 is much greater than the initial concentration of HCl, we can assume that the concentration of NH3 does not change significantly during the titration.
\(Kb = x^2 / 0.050\\x = \sqrt{Kb * 0.050} = 1.3 * 10^{-3} M\)
The concentration of OH- is equal to \(1.3 * 10^{-3} M\), so we can calculate the pH:
\(pH = 14 - pOH = 14 - (-log[OH-]) = 11.89\)
Therefore, the pH at the start of the titration is 11.89.
B. 20 mL of titrant
After adding 20 mL of 0.025 M HCl, the volume of the solution is 50 mL (30 mL NH3 + 20 mL HCl). The moles of HCl added is:
moles of HCl = volume x concentration = 0.020 L x 0.025 mol/L = 5 x 10^-4 mol
Since the reaction is a 1:1 reaction, the moles of NH3 remaining is equal to the moles of HCl added.
concentration of NH3 = moles of NH3 / volume of NH3 = (0.050 mol/L x 0.030 L - 5 x 10^-4 mol) / 0.030 L = 0.048 mol/L
Since the concentration of NH3 has decreased, we need to recalculate the concentration of OH- using the new concentration of NH3:
\(Kb = [NH4+][OH-] / [NH3]\\1.8 * 10^{-5} = x^2 / (0.048 - x)\)
Solving for x, we get:
\(x = 1.3 * 10^{-3} M\)
The concentration of OH- is still \(x = 1.3 * 10^{-3} M\), so we can calculate the pH:
pH = 14 - pOH = 14 - (-log[OH-]) = 11.89
Therefore, the pH after adding 20 mL of titrant is still 11.89.
Similarly for C. 59.1 mL of titrant
The pH after adding 59.1 mL of titrant is 8.45.
D. 60 mL of titrant
The pH after adding 60 mL of titrant is 8.45.
E. 71.4 mL of titrant
The pH after adding 71.4 mL of titrant is 7.98.
F. 73.4 mL of titrant
The pH after adding 73.4 mL of titrant is 8.95.
For more question on pH click on
https://brainly.com/question/12609985
#SPJ11
Arrange the following ions in order of increasing ionic radius: K+, p3-, S2-, Cl". Select one: O a. Kt
Answers
The correct arrangement of ions in increasing ionic radius is as follows:
p3- < S2- < Cl- < K+
Therefore, the correct option is:
b. p3-, S2-, Cl-, K+
In general, the ionic radius increases as you move from right to left across a period and from top to bottom within a group on the periodic table. Therefore, the arrangement of ions in increasing ionic radius is p3- < S2- < Cl- < K+.
To learn more about Ionic Radius from the given link
https://brainly.com/question/8137711
#SPJ4
I need some help with this I would really appreciate if you could help out. Thank You

Answers
The type of chemical reaction occuring in the table above is as follows:
DecompositionCombination CombustionSingle replacementDouble displacementWhat is a chemical reaction?A chemical reaction is a process involving the breaking or making of interatomic bonds, in which one or more substances are changed into others.
There are several types of chemical reactions as illustrated in the equation given in the above table. They are as follows:
Decomposition reaction; This involves the breaking down of a large substance into its subsequent parts. Combination reaction: This involves the joining of two or more chemical elements to become one compound. Combustion reaction: It is a process wherein a fuel is combined with oxygen, usually at high temperature, releasing heat.Displacement reaction: In this reaction, one or more element is replaced in a compound by another in a chemical reaction.Learn more about chemical reaction at: https://brainly.com/question/22817140
#SPJ1
How could IR spectroscopy distinguish between 1-hexyne, 2-hexyne, and 3-hexyne? Check all that apply.
2-Hexyne will show neither the absorption band at ∼3300cm−1 nor the one at ∼2100cm−1 (there is no change in dipole moment when the C≡C stretches).
1-Hexyne will show absorption bands at ∼3300cm−1 for a hydrogen bonded to an sp carbon and at ∼2100cm−1 for the triple bond.
3-Hexyne will show the absorption band at ∼2100cm−1 but not the one at ∼3300cm−1.
3-Hexyne will show neither the absorption band at ∼3300cm−1 nor the one at ∼2100cm−1 (there is no change in dipole moment when the C≡C stretches).
1-Hexyne will show the absorption band at ∼2100cm−1 but not the one at ∼3300cm−1.
2-Hexyne will show absorption bands at ∼3300cm−1 for a hydrogen bonded to an sp3 carbon and at ∼2100cm−1 for the triple bond.
1-Hexyne will show neither the absorption band at ∼3300cm−1 nor the one at ∼2100cm−1 (there is no change in dipole moment when the C≡C stretches).
1-Hexyne will show absorption bands at ∼2100cm−1 for a hydrogen bonded to an sp carbon and at ∼3300cm−1 for the triple bond.
2-Hexyne will show the absorption band at ∼2100cm−1 but not the one at ∼3300cm−1.
Answers
1-Hexyne will show absorption bands at ∼3300cm−1 for a hydrogen bonded to an sp carbon and at ∼2100cm−1 for the triple bond.
2-Hexyne will show absorption bands at ∼3300cm−1 for a hydrogen bonded to an sp3 carbon and at ∼2100cm−1 for the triple bond.
3-Hexyne will show the absorption band at ∼2100cm−1 but not the one at ∼3300cm−1.
IR spectroscopy is a technique that measures the vibrational modes of molecules. Each molecule has a unique set of vibrational modes that correspond to different types of bonds within the molecule. In the case of alkynes, the C≡C triple bond is a strong bond that will produce a characteristic peak in the IR spectrum at around 2100 cm^-1. The presence or absence of other peaks will depend on the specific structure of the molecule.
For 1-hexyne, the hydrogen bonded to the sp carbon will produce a peak at around 3300 cm^-1 due to the stretching of the C-H bond. The C≡C triple bond will produce a peak at around 2100 cm^-1.
For 2-hexyne, the hydrogen bonded to the sp3 carbon will produce a peak at around 3300 cm^-1 due to the stretching of the C-H bond. The C≡C triple bond will produce a peak at around 2100 cm^-1.
For 3-hexyne, the C≡C triple bond will produce a peak at around 2100 cm^-1, but there is no hydrogen bonded to an sp carbon, so there will be no peak at around 3300 cm^-1.
To know more about absorption bands click here:
https://brainly.com/question/31115658
#SPJ11
can someone pls help! If I have 17 moles of gas at a temperature of 67°C, and a pressure of 5.34 atmospheres, what is the volume of the gas? Enter the unit of your answer here. Enter it as a symbol, not words spelled out.
Answers
Answer: Therefore, to convert the moles of gas to pressure, the scientist must know the volume and temperature of the gas, in addition to the number of moles of gas. The pressure is then given by P = nRT / V. Convert the volume and temperature to units of liters and Kelvin, respectively, if necessary
the solubility of solute in a solvent in a solid solution is governed by hume-rothery rules. the solubility is more if:
Answers
If solute has low valence radii are much smaller than that of solvent, then the solubility will be higher.
How do you increase the solubility of a substance?Increase in the temperature of the solution increases the solubility of a solute. For example, a high amount of sugar will dissolve in warm water as compared to cold water.
Solubility of solute in a solvent in a solution is control by Hume Rothery rules. The solubility is more if solute has low valence radii and the solute are smaller than the solvent.
Solubility is defined as the maximum amount of a substance that will dissolve in a specific amount of solvent at a particular temperature. There are two main factors that affect solubility of a solution i.e. temperature and pressure.
So we can conclude that If solute has low valence radii are smaller than solvent.
Learn more about solubility here: https://brainly.com/question/23946616
#SPJ1
in general, how do the periodic properties of the d-block elements compare with those of the main - group elements?
Answers
The periodic properties of the d-block elements differ from the main group elements in that they are less sensitive and less reactive.
The periodic table is divided into blocks; s-block, p-block, f-block, and d-block. The d-block elements are also known as transition metals.
The s and p-block elements are known as the main group elements. Compared to these, the d-block elements have some different properties because of their partially filled d-orbitals.
However, the d-block elements still have many similar properties. These elements can still displace hydrogen from dilute acid and some of them can react with water under appropriate conditions.
The first row of these transition metals are found to be more reactive than the second and third row. However, they are not as reactive as the s-block and p-block elements.
To learn more about d-block elements; click here:
https://brainly.com/question/12346980
#SPJ4
Atoms lose, gain, or share valence electrons to satisfy ________.
Options
1.Hund’s Rule
2.The rule of Nines
3.the Heisenburg Uncertainty Principle
4.the Octet Rule
Answers
The Octet Rule requires all atoms in a molecule to have 8 valence electrons--either by sharing, losing or gaining electrons--to become stable. For Covalent bonds, atoms tend to share their electrons with each other to satisfy the Octet Rule.
we can consider a liquid-liquid extraction to be efficient if >90% of the desired compound can be recovered. presuming (i) the desired compound is soluble in the organic solvent and (ii) we use equal volumes of the organic solvent and water, what is the minimum value of the partition coefficient (k) to get an efficient extraction with only one extraction step (i.e. only mixing the organic solvent and water once, without further extractions using fresh portions of organic solvent)?
Answers
The minimum value of the partition coefficient (k) to get an efficient extraction with only one extraction step is 1.8.
To answer your question, we need to understand the concept of partition coefficient (k). Partition coefficient (k) is the ratio of the concentration of a solute in the organic phase to its concentration in the aqueous phase at equilibrium. It is a measure of the solubility of a solute in a particular solvent system.
Now, to get an efficient extraction with only one extraction step, we need to ensure that more than 90% of the desired compound is recovered. Given that the desired compound is soluble in the organic solvent and we use equal volumes of the organic solvent and water, the minimum value of the partition coefficient (k) can be calculated using the following formula:
k = [concentration of the desired compound in the organic phase] / [concentration of the desired compound in the aqueous phase]
To achieve an efficient extraction with only one extraction step, we need to ensure that more than 90% of the desired compound is extracted into the organic phase. This means that the concentration of the desired compound in the organic phase should be at least 90% of the initial concentration of the compound. Assuming equal volumes of the organic solvent and water are used, this can be represented as:
[concentration of the desired compound in the organic phase] >= 0.9 x [initial concentration of the desired compound]
Similarly, the concentration of the desired compound in the aqueous phase can be represented as:
[concentration of the desired compound in the aqueous phase] = [initial concentration of the desired compound] / 2
Substituting these values in the formula for k, we get:
k >= (0.9 x [initial concentration of the desired compound]) / ([initial concentration of the desired compound] / 2
Simplifying the expression, we get:
k >= 1.8
In summary, for an efficient extraction with only one extraction step, we need to ensure that the desired compound is soluble in the organic solvent and the partition coefficient (k) is equal to or greater than 1.8.
To know more about organic solvent visit :
https://brainly.com/question/17406963
#SPJ11
PLZ HELP PLZ SOMEONE PLZ How old do u need to work as a secret agent
Answers
I THINK 18 OR 21 I AM SORRY IF I AM WRONG BRO
An unknown metal has a mass of 51.842 grams. when placed in a graduated cylinder, the volume of water rose from 17.1 ml to 19.8 ml. what is the identity of the metal?
Answers
The density of the metal, given that it has a mass of 51.842 grams is 19.2 g/mL
How to determine the densityFirst, we shall determine the volume of the metal. This can be obtained as follow:
Volume of water = 17.1 mL Volume of water + metal = 19.8 mL Volume of metal =?Volume of metal = (Volume of water + metal) - volume of water
Volume of metal = 19.8 – 17.1
Volume of metal = 2.7 mL
Finally, we shall determine the density of the metal. This is illustrated below:
Mass of metal = 51.842 gramsVolume of metal = 2.7 mL Density of metal = ?Density = mass / volume
Density of metal = 51.842 / 2.7
Density of metal = 19.2 g/mL
From the above calculations, we can conclude that the density of the metal is 19.2 g/mL
Learn more about density:
https://brainly.com/question/952755
#SPJ1
If 67.5 mol of an ideal gas occupies 71.5 L at 47.00 °C, what is the pressure of the gas?
Answers
n = 67.5 mol
V = 71.5 L
T = 47°C + 273 K = 320 К
R = 0.0821
P = (67.5•0.0821•320) / 71.5 = 24.8 atm
Answer:
24.8 atm
Explanation:
For this problem, first identify all of the given quantities, and the requested quantity:
Amount: 67.5 mol
Volume: 71.5 L
Temperature: 47.00 °C
Pressure: ??
Since we are given that the gas is an "ideal gas", we can apply the ideal gas law, which relates all of the above quantities:
\(PV=nRT\), where
P is the pressure, V is the Volume, n is the amount in moles, R is the ideal gas constant (the value of R is different depending on which units are used for Pressure and Volume), and T is the Temperature measured in Kelvin.For Pressure in units of "atmospheres" and Volume in units of "Liters", \(R=0.0821\frac{L \cdot atm}{mol \cdot K}\)
Recall that to convert Celsius to Kelvin, one must add 273.15 or use the equation \(T_C+273.15=T_K\)
So \(T_K=(47.00 + 273.15)K=320.15K\), which is the temperature that we'll need for the Ideal Gas Law.
Since the Pressure is the unknown quantity, we can isolate P in the equation before substituting the known values:
\(PV=nRT\)
divide both sides by V...
\(P=\dfrac{nRT}{V}\)
Now, substitute and calculate:
\(P=\dfrac{(67.5~mol)(0.0821\frac{L \cdot atm}{mol \cdot K})(320.15~K)}{(71.5~L)}\)
\(P=24.8138638111888~atm\)
Accounting for significant digits, \(P=24.8~atm\)
Choose the proper whole-number coeffi-
cients for each substance to yield a balanced
equation.
D
1. 2, 1, 2
2. 1, 1, 1
3. 1, 2, 1
4. 2, 1,1
Answers
The proper whole number coefficient for each substance to yield a balanced equation is option 1 that is 2, 1, 2.
The proper whole number coefficient for each substance to yield a balanced equation is option 1 that is 2, 1, 2. Balancing a chemical reaction is done to find out the overview of that reaction and to find the answers related to that equation. like we can tell many things about the equation just by knowing the coefficients of the terms in that equation. we can tell about the percentage and existence of the products and reactants in the equation. if we know the coefficients of the elements and products we can tell about the mole of the reactants and products in the chemical equation. By this information, we can consider that the proper whole number coefficient for each substance to yield a balanced equation is option 1 that is 2, 1, 2.
Learn more about Balanced equation:
brainly.com/question/28294176
#SPJ4
\(\huge\bold\red{{HELP}}\)
Help me ireally need it cause i will pass it tommorow
![[tex]\huge\bold\red{{HELP}}[/tex]Help me ireally need it cause i will pass it tommorow](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/2tUQoXf4WRhC7QVE7Qg7SHXXoPhsbwat.jpeg)
Answers
Answer:
Question 1 :
• A compound is ionic if it is made up of a metal or a cation (+) and a non metal or anion (-)
Question 2 :
• While naming ionic compounds, follow the formula → "metal" + "non-metal ending with ide "
• i.e; Sodium Chloride:
\({ \rm{Na {}^{ + } _{(aq)} + Cl {}^{ - } _{(aq)} \: \dashrightarrow \: NaCl _{(aq)}} \: \: \: \: \: \: \: \: } \\ { \rm{sodium + chloride \: \dashrightarrow \: sodium \: chloride}} \)
Question 3 :
• The answer above that question is perfect.
Question 4 :
1 atom → Mono. But it is highly recommended to ignore it
2 atoms → DI
3 atoms → TRI
4 atoms → TETRA
5 atoms → PENTA ( such as pentaoxide )
7 atoms → HEPTA ( such as heptaoxide )
Question 5 and 6:
Are perfectly answered.
How do instantaneous speed and average speed differ?
Instantaneous speed happens all the time, but average speed only happens once per hour.
Instantaneous speed is always faster than average speed.
Instantaneous speed is the speed of an object at a specific time, but average speed is the total distance over the total time.
Instantaneous speed is always slower than average speed.
Answers
Answer:
Option 3
Explanation:
Option 3: Instantaneous speed is the speed of an object at a specific time, but average speed is the total distance over the total time. This is why they are called instantaneous and average, because instantaneous is at any one instance and average is the average speed it was moving over the total time which is calculated by the total distance/total time.
Hope this helped!
Answer:
Average Speed
Explanation:
T or F: All electrons are alike

Answers
Answer:
True
Explanation:
Every electron in the universe has exactly the same mass, and exactly the same charge.
IUPAC name for CH2(OH)-CH2-CH2(OH)
Answers
The IUPAC name for CH2(OH)-CH2-CH2(OH) is 1,2,3-propanetriol. It is also commonly known as glycerol or glycerin.
Explanation:
The IUPAC name for a molecule is a systematic way of naming a compound based on the rules set by the International Union of Pure and Applied Chemistry (IUPAC). In the case of CH2(OH)-CH2-CH2(OH), the IUPAC name is based on the longest carbon chain, which is a three-carbon chain. The -OH groups attached to the carbon chain are named as substituents, with the prefix "hydroxy-" indicating the presence of an -OH group. The first carbon atom in the chain is numbered as "1," and the -OH groups are assigned the lowest possible numbers.
Therefore, the IUPAC name for CH2(OH)-CH2-CH2(OH) is 1,2,3-propanetriol. It is named as propanetriol because it contains a three-carbon chain and three -OH groups. It is also commonly known as glycerol or glycerin and is an important compound used in many industries, including food, cosmetics, and pharmaceuticals.
While you are conducting your experiment, you notice that the ball bounces at a different height and in a different direction for each trial. You look at the ground and see it is uneven. What could you do?
Answers
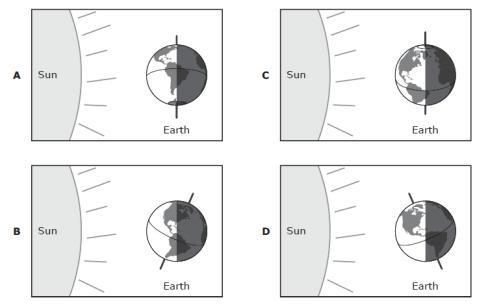
Which of the following diagrams represents the winter in the southern hemisphere?
Answers
Answer:
Left position
If this don't help i am sorry but i tried
Explanation:
The figure is representing the motion of revolution of the Earth around the Sun.
This motion lasts approximately 365 days (one year). Since the axis of rotation of the Earth is tilted (by about 23 degrees from the vertical), the amount of light received by different parts of the Earth at the different part of the year is different.
In fact, we observe 4 "extreme" cases:
- Situation at the bottom: here the situation is symmetrical in the two hemispheres, which receive an equal amount of light. So, we have 12 hours of daylight and 12 hours of dark at every point on Earth - this is called equinox, and it occurs in September
- Situation on the right: here we see that the Southern Hemisphere receive much more light than the Northern Hemisphere - so the Southern Hemisphere has more hours of sunlight per day. This is called winter solstice, and it occurs in December - in this case, it is summer in the Southern Hemisphere and winter in the Northern Hemisphere
- Situation at the top: here the situation is symmetrical in the two hemispheres, which receive an equal amount of light. So, we have 12 hours of daylight and 12 hours of dark at every point on Earth - this is called equinox, and it occurs in March
- Situation on the left: here we see that the Northern Hemisphere receive much more light than the Southern Hemisphere - so the Northern Hemisphere has more hours of sunlight per day. This is called summer solstice, and it occurs in June - in this case, it is summer in the Northern Hemisphere and winter in the Southern Hemisphere
how would your calculations of the concentration of [fescn]2 been affected if the cuvette you used had a 1.5 cm path length rather than the 1.0 cm value you were told to use?
Answers
The increased distance across the cell will result in an increase absorbance reading.
The concentration of \([Fescn]_2\) would be affected if the cuvette had a 1.5 cm path length rather than the 1.0 cm value used.Since the absorbance of a sample is proportional to the concentration of a sample (as described by the Beer-Lambert law), increasing the path length of the cuvette would result in a decrease in absorbance. This means that the concentration of the sample would be lower than if the 1 cm path length was used. In other words, the concentration of \([Fescn]_2\)would be lower if the cuvette had a 1.5 cm path length than if it had a 1.0 cm path length.
learn more about cuvette Refer:brainly.com/question/29385690
#SPJ1
(25 POINTS AND BRAINLIEST)What is the volume of 2 mol of chlorine gas at STP?
2.0 L
11.2 L
22.4 L
44.8 L
Answers
Answer:
44.8
Explanation:
According to standard temperature and pressure and ideal gas equation , the volume of 2 mole of chlorine gas at STP is 44.8 liters.
What are standard temperature and pressure conditions?Standard temperature and pressure are defined as a standard set of conditions required for experimental measurements which are established to allow comparison between different sets of data.
Standards which are commonly used are those International Union of pure and applied chemistry and national institute of standards and technology.These are not universally accepted standards but are the ones which are commonly used.
Standard conditions of pressure and temperature are necessary to define standard reference conditions used to express volumes of liquids and gases.
These values are important to physicists, chemists ,engineers ,pilots and navigators.On substituting values in ideal gas equation, volume= 2×8.314×273/1=44.8 liters.
Thus, the volume of 2 mole of chlorine gas at STP is 44.8 liters.
Learn more about standard temperature and pressure , here:
https://brainly.com/question/29129606
#SPJ6
Name the nutrient present in cooked rice , a boiler eggs
Answers
Explanation:
cooked rice
nutrient
carbohydrate.
Boiler eggs
nutrient
Protein.
Explain the large variation in boiling temperatures, given the small range in Me values.

Answers
Answer:
32.0 is the answer which is divide able to 65 or -85
What is the formula for Iron(IV) phosphite
Answers
Answer:
The answer is Iron phosphide (Fe2P)