The table shows several gases their molar masses and their densities at STP
Answers
The formula expressed as D= M/V is used at the STP where M designates for the molar mass of the substance and V is the molar volume of a gas.
The molar mass of the chemical compound can be defined as the ratio between the mass and the amount of substance of any sample of the given compound. At STP it is clear that 1 mole of a gas occupies the volume of 22.4 L. The density of gas is expressed as the ratio of its mass to volume of the chemical compound. Using the expression of density we can calculate the mass of 22.4 L of gas which corresponds to the mass of 1 mole of a gas which is taken as its molar mass.
The standard Temperature and Pressure is also called as STP predicts the molar volume is the volume occupied by one mole of a chemical element or occupied by a chemical compound. This can be usually calculated by dividing the molar mass (M) of the chemical compound by the mass density (ρ) of the chemical compound.
To learn more about STP
https://brainly.com/question/2783971
#SPJ4
The complete question is,
What is the relation between molar mass and STP ?
Related Questions
A hurricane is MOST LIKELY to occur in an area
Answers
Answer:
Western Pacific, such as the Philippines, Guam, southeast Asia (including China and Taiwan) and Japan.
Explanation:
answer this step by step pls
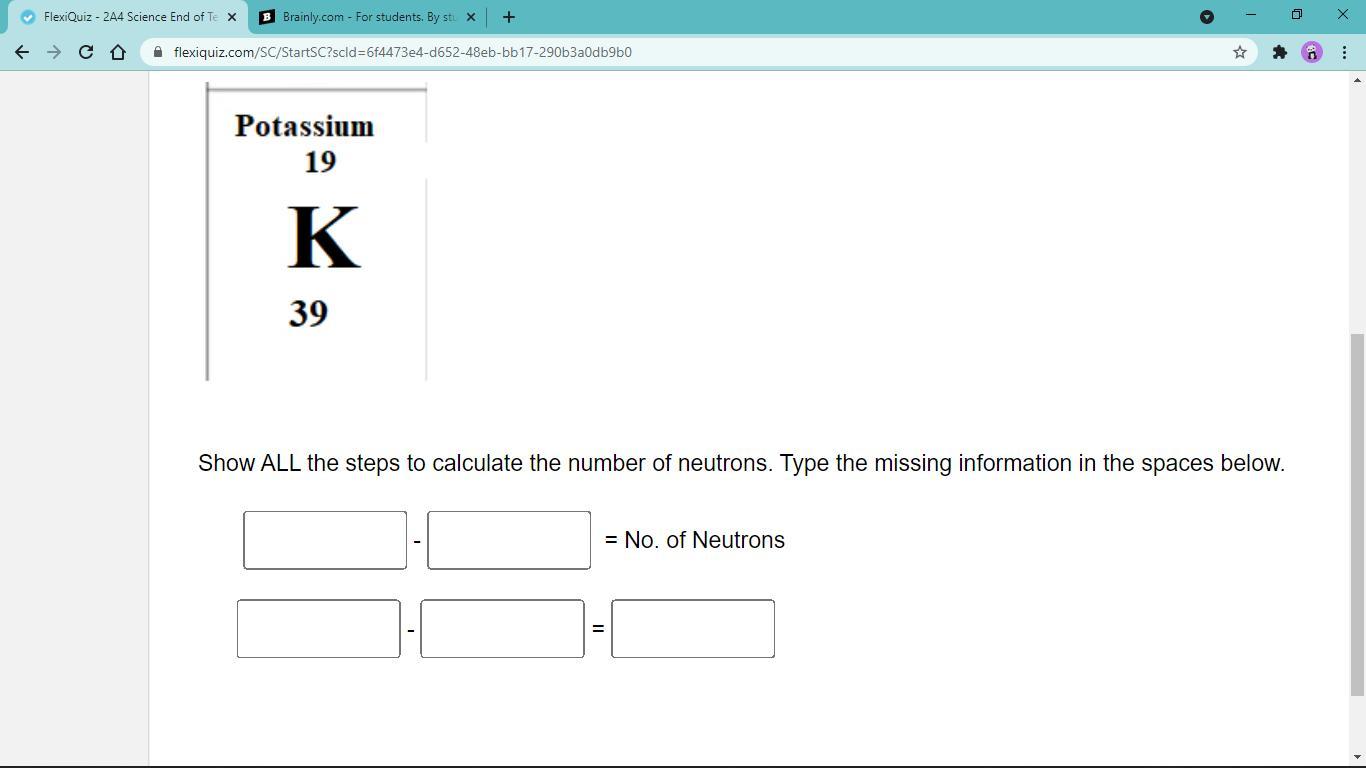
Answers
Answer:
neutrons = mass number - atomic number
. = 39-19 =20
An old refrigerator is rated at 500 W how many kilowatt hours of electric energy what does refrigerator use in 30 days assume the refrigerator is running 12 hours per day
Answers
The refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
To calculate the kilowatt-hours (kWh) of electric energy used by the refrigerator in 30 days, we need to multiply the power rating by the total running time.
Given:
Power rating of the refrigerator = 500 W
Running time per day = 12 hours
Number of days = 30
First, we need to convert the power rating from watts to kilowatts:
Power rating = 500 W / 1000 = 0.5 kW
Next, we calculate the total energy used in kilowatt-hours (kWh) over the 30-day period:
Energy used = Power rating × Running time × Number of days
Energy used = 0.5 kW × 12 hours/day × 30 days
Energy used = 180 kWh
Therefore, the refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
For more question on energy
https://brainly.com/question/29339318
#SPJ8
8. Some people practice brining their turkey. This means they let it soak in a solution overnight. The solution diffuses into the turkey. This means that the turkey is placed into a ___________________ solution.
Answers
Some people practice brining their turkey. This means they let it soak in a solution overnight. The solution diffuses into the turkey. This means that the turkey is placed into a hypertonic solution.
What is a hypertonic solution?A hypotonic solution is described as a solution that has lower osmotic pressure than another solution to which it is compared.
The concept of tonicity helps us understand that the saltwater we use to brine the turkey is typically considered hypertonic solution because it has a greater concentration of solutes than the liquid found inside the cells.
Learn more about hypertonic solutions at: https://brainly.com/question/4237735
#SPJ1
how do you find an electron affinity?
Answers
Answer:
Explanation:
Electron affinity is the measure of an atom 's tendency to form a negative ion. Electron affinity can be measured by the amount of energy released when an electron is added to an atom to form a negative ion. When forming a negative ion, an atom undergoes the following reaction:: atom + electron (e -) -> negative ion + energy
what are electrons. in your own words
Answers
Answer:
Electrons are the smallest of the particles that make up an atom, and they carry a negative charge.
Answer:
Electrons are the smallest subatomic particles that make up an atom. They have a mass of 1/1836 amu's or 0 amu's (no mass) Electrons are located in the electron cloud and have a negative charge.
Explanation:
Hope that helps
What is the percent composition by mass of sulfur in the compound, mgso4? the formula mass for magnesium sulfate is 120 g/mol.
Answers
Percentage composition by mass of sulfur in MgSO₄ is 28.33% if the formula mass of magnesium sulfate is 120g/mol.
Formula mass and sub-atomic mass are two qualities that express the size of a particle.
The formula mass (recipe weight) of a particle is the amount of the nuclear loads of the molecules in its exact recipe.
The sub-atomic mass (sub-atomic weight) of a particle is its typical mass as determined by including the nuclear loads of the atoms in the atomic recipe.
Formula mass of MgSO₄=120g/mol
Now, we know that atomic mass of sulfur is 32g. Here, we have 1 atom of sulfur. So, total atomic mass of sulfur =32 × 1 g/mol.=32g/mol.
Now, percentage composition of sulfur = (32/120) × 100
=>percentage composition of sulfur = 3200/120
=>percentage composition of sulfur=320/12=28.33%
Hence, percentage composition by mass of sulfur is 28.33%
To know more about percentage composition, visit here:
https://brainly.com/question/14899075
#SPJ4
this metal is more reactive than lithium and magnesium but less reactive than potassium. this elem is
Answers
Calcium is the element that is more reactive than lithium and magnesium but less reactive than potassium.
Calcium is an alkaline earth metal that is highly reactive and a silvery-white solid at room temperature. It is the 5th most abundant element on Earth's crust and the third most abundant metal after aluminum and iron. Calcium is more reactive than lithium and magnesium but less reactive than potassium.
Calcium reacts with water to produce hydrogen gas and calcium hydroxide. It also reacts with oxygen in the air to form a thin oxide layer that protects the metal from further oxidation. Calcium is widely used in the production of alloys, cement, and fertilizers. It is also an essential element in the human body, where it plays a crucial role in bone and teeth formation, muscle contraction, and nerve function.
Learn more about magnesium here:
https://brainly.com/question/8351050
#SPJ11
Round 0.002346 to 3 significant figures.
Answers
Answer:
0.00235
Explanation:
Zeros that come before any non-zero digits are never significant.Zeros that are in between any non-zero digits are always significant.Zeros that come after any non-zero digit are ONLY significant if a decimal point in present somewhere in the number. (In this case there is a decimal point, but there aren't any zeros at the end of this number.)The zeros at the beginning aren't significant, so include those zeros in your answer. The 6 at the end in greater than 5 so that rounds the 4 to up one number (In this case, it's 5).
This gives you 0.00235
a 1.0-l buffer solution initially contains 0.25 mol of nh3 and 0.25 mol of nh4cl. in order to adjust the buffer ph to 8.75, should you add naoh or hcl to the buffer mixture? what mass of the correct reagent should you add?
Answers
1.0 L buffer solution initially contains 0.25 mol of NH₃ and 0.25 mol of NH₄Cl. to adjust the buffer pH to 8.75, we should add HCl . The mass of reagent is 4.51 g.
given that :m
volume of buffer solution = 1 L
number of moles of NH₃ = 0.25 mol
number of moles of NH₄Cl = 0.25 mol
pH = 8.75
Molarity of NH₃ and NH₄Cl is as follows:
Molarity = number of moles / volumes in L
Molarity = 0.25 / 1
Molarity = 0.25 M
pKa for NH₄Cl = 9.25
now the pH expression is given as :
pH = pKa + log ( 0.25 / 0.25 )
pH = 9.25 + log 1
pH initial = 9.25
now from the question pH needs to be 8.75. that means we have to reduce the pH.
new concentration A⁻ = 0.25 - x
HA= 0.25 + x
pH = pKa + log ( base / acid )
8.75 = 9.25 + log ( (0.25 - x ) / ( 0.25 + x))
-0.5 = log ( (0.25 - x ) / ( 0.25 + x))
0.316 = ( (0.25 - x ) / ( 0.25 + x))
x = 0.124 M
to decrease the pH , we have increase the H⁺ ion concentration. so we should add HCl in a buffer solution.
number of moles of hydronium ion = 0.124 × 1
= 0.124 mol
molar mass of HCl = 36.45 g/mol
mass of HCl = moles × molar mass
= 0.124 × 36.45
= 4.51 g mass of reagent.
Thus, 1.0 L buffer solution initially contains 0.25 mol of NH₃ and 0.25 mol of NH₄Cl. to adjust the buffer pH to 8.75, we should add HCl . The mass of reagent is 4.51 g.
To learn more about buffer solution here
https://brainly.com/question/19952944
#SPJ4
intramolecular bonding of polypeptide chains produces numerous ____________ and beta sheets.
Answers
Intramolecular bonding of polypeptide chains produces numerous α-helices and β-sheets.
Polypeptide chains, which are the backbone of proteins, can fold and interact with each other through various intramolecular bonds. Two common types of secondary structures formed by these interactions are α-helices and β-sheets.
α-helices are formed by the twisting of the polypeptide chain into a helical structure held together by hydrogen bonds between the amino acid residues. The hydrogen bonds form between the carbonyl group of one amino acid and the amino group of another, creating a stable helical conformation.
β-sheets, on the other hand, are formed by the alignment of multiple polypeptide strands held together by hydrogen bonds between adjacent strands. The hydrogen bonds form between the carbonyl and amino groups of different strands, creating a pleated sheet-like structure.
To know more about polypeptide chains, refer here:
https://brainly.com/question/29794354#
#SPJ11
Is water solvent or solute
Answers
Answer:
Water is a solvent.
Explanation:
What is solvent? Solvent is substance, ordinarily a liquid, in which other materials dissolve to form a solution.
Which balanced equation represents a neutralization reaction?
H₂SO4 + 2LIOH → Li2SO4 + 2H2O .
BaCl2 + Cu(NO3)2 → Ba(NO3)2 + CuCl2
2KCIO3 → 2KCI+ 302
Mg + NiCl2 → MgCl2 + Ni
Answers
The answer is A: H₂SO4 + 2LIOH → Li2SO4 + 2H2O
Answer:
H₂SO4 + 2LIOH → Li2SO4 + 2H2O
Explanation:
The balanced equation that represents a neutralization reaction is:
H₂SO4 + 2LIOH → Li2SO4 + 2H2O
This is a neutralization reaction because the acidic hydrogen ions (H+) in sulfuric acid (H₂SO4) react with the basic hydroxide ions (OH-) in lithium hydroxide (LiOH) to form water (H2O) and a salt (Li2SO4). The resulting solution will be neutral as the acid and base have neutralized each other.
A mixture consists of three gases, A, B, and C. The partial pressure of A is 5.1 Pa, of B is 1.5 Pa, and of C is 1.2 Pa. The total pressure of the mixture is ____________________ Pa.
7.8
Answers
A, B, and C are the three gases that make up a mixture. 5.1 Pa, 1.5 Pa, and 1.2 Pa respectively make up the partial pressures of A, B, and C. 7.8 Pa is the mixture's total pressure.
What in science is a mixture?Three or more distinct, pure components come together to form a combination. Hazardous and homogeneous mixtures are the two types of mixtures. While homogeneous mixtures have a constant appearance throughout, heterogeneous mixtures have unique features.
What are a mixture and an illustration?A mixtures is a chemical in chemistry that is comprised of two or more simpler components. These materials could be chemical components or elements. A mixture of liquid, sediments, or gases may be produced. As an illustration, adding sugar to water results in a
To know more about mixture visit:
https://brainly.com/question/24898889
#SPJ4
ethanol, , is mixed with gasoline and sold as gasohol. use the following to calculate the grams of ethanol needed to provide 575 kj of heat.
Answers
21.47 g of ethane is needed to provide the heat . To calculate the grams of ethanol needed to provide 575 kJ of heat, the mass of ethanol used can be calculated using the following formula.
Mass of ethanol = (heat of combustion/heat of combustion per gram of ethanol) * volume of gasohol
The combustion of ethanol generates 1234 kJ of energy per mole. Therefore, the heat of combustion of ethanol can be calculated as follows:
Heat of combustion = (1234 kJ/mol)(1 mol)
Heat of combustion = 1234 kJ/mol
Now, the heat of combustion per gram of ethanol can be determined by dividing the heat of combustion by the molar mass of ethanol.
Heat of combustion per gram of ethanol = Heat of combustion/molar mass of ethanol
Heat of combustion per gram of ethanol = (1234 kJ/mol)/(46.07 g/mol)
Heat of combustion per gram of ethanol = 26.77 kJ/g
To calculate the mass of ethanol required to provide 575 kJ of heat, the following formula can be used:
Mass of ethanol = (heat of combustion/heat of combustion per gram of ethanol) * volume of gasohol
Mass of ethanol = (575 kJ/26.77 kJ/g) * 1 L
Mass of ethanol = 21.47 g
Therefore, 21.47 g of ethanol is needed to provide 575 kJ of heat.
To know more about the ethanol https://brainly.com/question/25002448
#SPJ11
Using the periodic table, calculate the number of protons, neutrons, and electrons for Calcium, Argon, and Bromine.(Ca,Ar,Br)
Answers
Answer:
Calcium has 20 protons 26 neutrons and 20 electrons.
argon has 18 protons
22 neutrons and 18 electrons
Bromine has 35 protons 44 neutrons and 35 electonrs
Explanation:
good luck with your homework hope I helped
true or false: an atom with more neutrons and more electrons than protons can be expected to have no net charge.
Answers
An atom with more neutrons and more electrons than protons can be expected to have no net charge. This statement is False.
Matter atoms are electrically neutral because their nuclei have the same amount of protons as electrons around them. The separation of part of the negative charge of neutral atoms is involved in electric current and charged objects. Neutrons do not have a negative or positive charge. As a result, they have no net electric charge.
An atom is made up of a positively charged nucleus surrounded by one or more negatively charged electrons. Because the positive and negative charges are equal, the atom has no overall charge; it is electrically neutral.
To learn more about charge on atoms, here
https://brainly.com/question/14548352
#SPJ4
How do weathering and deposition differ? Weathering breaks down rocks; deposition leaves them in new places. Weathering has to do with air; deposition has to do with plants. Weathering occurs only in summer; deposition occurs year-round. Weathering can be chemical or physical; deposition is only chemical
Answers
Answer:
A. Weathering breaks down rocks; Deposition leaves them in new places.
Explanation:
Weathering is basically the complete process of rocks breaking apart. In contrast, deposition is when the rocks are moved and carried away from their original place and put in new locations.
Answer:
a
Explanation:
What does the mass of an object depend on?
Answers
Answer:
the mass of an object depends on inertia greater is the inertia greater will be the mass or vice versa
Explanation:
hope it help ^_^
Mass is the most basic property of matter and it is one of the fundamental quantities. Mass is defined as the amount of matter present in a body. The SI unit of mass is the kilogram (kg). The formula of mass can be written as:
Mass = Density × Volume
The mass of a body is constant; it doesn’t change at any time. Only in certain extreme cases when a huge amount of energy is given or taken from a body, the mass may be impacted. For example, in a nuclear reaction, a tiny amount of matter is converted into a huge amount of energy, this reduces the mass of the substance.
There are various units for calculating mass, like, kilograms, grams, lbs, pounds, etc., but the SI unit of mass is "kilograms" or kg. Every unit of mass can be converted to other units by using a proper conversion formula without affecting the meaning and essence of the quantity to be measured.
HENCE,MASS OF AN OBJECT DEPENDS ON:
size of an atom or moleculesAnd number of atoms and molecules of the body.For more such examples follow this link:
https://brainly.in/question/22612207
A stock solution of sodium sulfate, Na2SO4 has a concentration of 1.00 M. The volume of this solution is 50 mL. What volume of a 0.25 M solution could be made from the stock solution?
Answers
We can make a 0.25 M solution with a volume of 200 mL from the stock solution.
How do you calculate the volume of a 0.25 M solution that could be made from the stock solution?We can use the dilution formula to fix this issue:
C1V1 = C2V2
where C1 and V1 represent the initial (stock) solution's concentration and volume, and C2 and V2 represent the end (diluted) solution's concentration and volume.
In order to write: "We want to determine the volume of a 0.25 M solution that can be created from the stock solution."
C2 = 0.25 M V2 =? where C1 = 1.00 M and V1 = 50 mL.
Using the dilution formula with these values as input, we obtain:
0.25 M x V2 = 1.00 M x 50 mL
If we simplify, we get:
50 mL = 0.25 M x V2
The result of dividing both sides by 0.25 M is:
V2 = 200 mL
To learn more about stock solution visit:
brainly.com/question/25256765
#SPJ1
the reaction below is spontaneous under standard conditions - true or false? cl2(g) fe2 (aq) → fe3 (aq) cl-(aq)
Answers
In chemistry, a spontaneous reaction is a type of reaction that occurs on its own without the need for external stimulus. This means that the reaction will occur without any activation energy. The driving force behind this type of reaction is the chemical potential of the reactants.
The reaction is spontaneous under standard conditions. The reaction given below is spontaneous under standard conditions -
Cl2(g) Fe2+(aq) → Fe3+(aq) Cl–(aq).
In chemistry, a spontaneous reaction is a type of reaction that occurs on its own without the need for external stimulus. This means that the reaction will occur without any activation energy. The driving force behind this type of reaction is the chemical potential of the reactants. It is the potential energy stored within the reactants that can be converted into kinetic energy of the products. The Gibbs free energy is used to determine if a reaction will be spontaneous or not under standard conditions.In the given reaction, the Cl2 and Fe2+ are the reactants. The Fe3+ and Cl– are the products. The Gibbs free energy change for the reaction is negative (-ve) (-2.2kJ/mol). This implies that the reaction is spontaneous under standard conditions. Hence, the given statement is true.
To know more about activation energy visit: https://brainly.com/question/28384644
#SPJ11
how much work must be done on the electron to move it to the negative plate if it is initially positioned 2.92 mm from the positive plate?
Answers
To calculate the amount of work required to move an electron to the negative plate, we need to know the potential difference between the plates. Let's assume that the potential difference is 100 volts. The formula for work is W = qV, where q is the charge and V is the potential difference.
The charge of an electron is 1.6 x 10^-19 coulombs. To move the electron from the positive plate to the negative plate, we need to do work against the electric field. The electric field is given by E = V/d, where d is the distance between the plates. So, the electric field is E = 100/2.92 x 10^-3 = 3.424 x 10^4 N/C.
The force on the electron is F = Eq, where q is the charge of the electron.
F = 3.424 x 10^4 x 1.6 x 10^-19 = 5.478 x 10^-15 N.
To move the electron from the positive plate to the negative plate, we need to work against this force. The distance over which we need to move the electron is d = 2.92 x 10^-3 m.
The work done on the electron is W = Fd = 5.478 x 10^-15 x 2.92 x 10^-3 = 1.599 x 10^-17 J.
Therefore, the amount of work required to move the electron to the negative plate is 1.599 x 10^-17 joules.
Learn more about electron here ;
https://brainly.com/question/18367541
#SPJ11
PLEASE HELP!!
George is writing an essay about the role of observation and inference in the development of the atomic theory. He wants to explain why it was more difficult to observe the presence of neutrons in atoms. Which statements should he include in his essay? Choose the two statements that apply.
A. While protons or electrons can be influenced by other charged particles, neutrons are not.
B. It was difficult to observe that neutrons were different than protons because the two particles respond to charge in the same way.
C. It was difficult to observe that neutrons were different than electrons because the two particles respond to charge in the same way.
D. Neutrons are held tightly together with protons in the nucleus, so scientists could not observe the behavior of neutrons independently.
Answers
Answer:
D and A
Explanation:
While protons or electrons can be influenced by other charged particles, neutrons are not.
and
Neutrons are held tightly together with protons in the nucleus, so scientists could not observe the behavior of neutrons independently.
Water will expand more than ____________.
door
air
juice

Answers
Answer:
door
Explanation:
door I guess..................
give the correct isotope symbol to identify and atom that contains 4 protons, 4 electrons, and 5 neutrons
Answers
The correct isotope symbol for the atom with 4 protons, 4 electrons, and 5 neutrons is 9Be.
The correct isotope symbol to identify an atom which contains 4 protons, 4 electrons, and 5 neutrons would be; 9Be
Here's a breakdown of the isotope symbol;
The atomic number is represented by the subscript, which indicates the number of protons. In this case, it is 4, so the symbol starts with the number 4.
The element symbol is Be, which represents the element beryllium.
The mass number will be the sum of the protons as well as neutrons. In this case, it is 4 protons + 5 neutrons = 9.
The mass number is usually written as a the superscript to left of the element symbol.
Therefore, the correct isotope symbol for the atom with 4 protons, 4 electrons, and 5 neutrons is 9Be.
To know more about isotope symbol here
https://brainly.com/question/29851218
#SPJ4
How do you find the unknown compound in organic chemistry?
Answers
The unknown compounds are among a restricted set of substances that are provided for you in ascending mp and bp order. Applying your experimental results to these lists will help you narrow down the list of potential chemicals.
Compounds can be recognized by two tests, including
1. Physical assessment
2. Chemical assessment
Physical assessment
This is dependent on outward manifestations and qualities including State, Color, Texture, Smell (odor), Taste, and Feel.
Physical characteristics such as solubility, crystalline or amorphous nature Refractive index, melting point, and boiling point
pH, conductivity, functional groups, and other chemical assessment criteria. utilizing analytical approaches such as spectroscopy analysis and chromatography analysis.
To know more about unknown compound visit
https://brainly.com/question/28456436
#SPJ4
calculate the mass of the product of 6.40g of magnesium with 1.32g of oxygen
Answers
Answer:
the mass of the product of 6.40 g of magnesium with 1.32 g of oxygen is 3.32 g.
Cathy is reading a science article about exercise. She learns that exercise
causes a person to breathe faster. The next part of the article has the title
"Heart Rate." In that part of the article, Cathy is sure she'll read about how
exercise causes the heart to beat faster. What active reading strategy is
Cathy using?
A. Monitoring and applying fix-up strategies
O B. Summarizing
O C. Making predictions
D. Making mental images
Answers
Pls help if you only know the correct answer! Thanks!!

Answers
Answer:
1. H=2, O=1
2. H=4, O=2
3. C=1, O=1
4.
a. reactants C=1, O=2
b. products C=1, O=2
5.
a. reactants H=4, O=2
b. products H=4, O=2
6
a. reactants C=1, O=1, H=4
b. products C=1, O=1, H=4
What is the frequency when the Energy is equal to 4.18 x 10-22 ?|
Answers
Answer:
6.31x10¹¹s⁻¹ = Frequency
Explanation:
To convert frequency to energy or vice versa we must use the equation:
e = h*f
Where e is energy in joules (4.18x10⁻²²J)
h is Planck's constant (6.626x10⁻³⁴Js)
Replacing:
4.18x10⁻²²J = 6.626x10⁻³⁴Js*f
6.31x10¹¹s⁻¹ = Frequency