The statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG Gibbs free energy of reaction and stands for the equilibrium constant. stands for the standard In each table, there may be one statement that is false because it contradicts the other three statements. If you find a false statement, check the box next to It. Otherwise, check the "no false statements box under the table. statement false? statement false? Ink>0
AH°
R<1
AG'>0
AG'>0
In > AH">TAS
no false statements
Answers
Answer:
see explaination
Explanation:
Please kindly check attachment for the step by step solution of the given problem

Related Questions
How many grams of ammonia(NH3) are produced from 6 moles of nitrogen. (Show all work)

Answers
Answer:
Mass = 204 g
Explanation:
Given data:
Mass of ammonia produced = ?
Number of moles of nitrogen = 6 moles
Solution:
Chemical equation;
N₂ + 3H₂ → 2NH₃
now we will compare the moles of nitrogen and ammonia.
N₂ : NH₃
1 : 2
6 : 2/1×6 = 12
Mass of ammonia;
Mass = number of moles × molar mass
Mass = 12 mol × 17 g/mol
Mass = 204 g
How much energy is required to raise the temperature of 4.0 g of mercury metal from 9.3 oC to 83.0 oC.
Answers
From the specific heat capacity of mercury, the amount of heat energy required to raise the temperature of 4.0 g of mercury metal from 9.3 °C to 83.0 °C is 77.792 J.
What is the specific capacity of mercury?The specific heat capacity of a substance is the amount of heat required to raise the temperature of a unit mass of the substance by one degree Celsius or kelvin.
The specific heat capacity of a substance is a constant that can be used to calculate the amount of heat required to raise the temperature of a given mass of a substance to any temperature.
The specific heat capacity of mercury is 0.140 J/g/k.
The formula for calculating specific heat capacity is given below:
Specific heat capacity, c = Δq/mΔT
where;
Δq = heat change
m = mass of the substance
ΔT = temperature change
The Heat required, Δq, will then be:
Δq = m * c * ΔT
Heat required, Δq = 4.0 * 0.140 * (83.0 - 9.3)
Heat required, Δq = 77.792 J
Lear more about specific heat capacity at: https://brainly.com/question/26866234
#SPJ1
Which of the following statements is true about the structure of an atom?
a: The electron cloud has all of the neutrons
b: Although the atomic nucleus contains most of the mass of the atom, it has none of the atom's electric charge.
c: The atomic nucleus contains most of the mass.
d: Although the atomic nucleus contains all of the atom's electric charge, most of the atom's mass is in the electron cloud.
Answers
Answer:
C: The atomic nucleus contains most of the mass.
Which amphibian organ has a high blood supply and many folds to increase surface area?
a. heart
b. stomach
c. lungs
d. brain
Answers
Answer:
lungs
Explanation:
What kind of ions does metal form?
* ionic bond
* covalent bond
* anions
* cations
* melting point
* boiling point
conductivity
* Polyatomic Ions
* Valence electron
* Metal
* Nonmetal
* Metaloids
Answers
Answer:
Option D: Cations
Explanation:
Do you need only one answer .
The decay of a living things allows chemical elements to be lost.
Answers
Answer: is is true
Explanation:
wally fluoride is an imaginary gaseous
compound with a molar mass of 314.2 g/mol.
(a) What is the density of wollmanium fluoride at 425 K
and 165 torr?
Answers
Answer:
\(\rho =1.96\frac{g}{L}\)
Explanation:
Hello there!
In this case, since this imaginary gas can be modelled as an ideal gas, we can write:
\(PV=nRT\)
Which can be written in terms of density and molar mass as shown below:
\(\frac{P}{RT} =\frac{n}{V} \\\\\frac{P}{RT} =\frac{m}{MM*V}\\\\\frac{P*MM}{RT} =\frac{m}{V}=\rho\)
Thus, by computing the pressure in atmospheres, the resulting density would be:
\(\rho = \frac{165/760 atm * 314.2 g/mol}{0.08206\frac{atm*L}{mol*K}*425K} \\\\\rho =1.96\frac{g}{L}\)
Best regards!
You have a balloon filled with hellum that has a volume of 4.91 cubic decimeters
at STP. Determine the mass of the hellum gas.
Answers
Answer:
32
Explanation:
because the number of heliem is your aswer
Answer:
so the balloon can fill up with air. If the balloon pops, then the air goes away.
Use the Rydberg Equation to calculate the energy in Joules of the transition between n = 7 and n = 3 for the hydrogen atom. Find the frequency in Hz of this transition if the wavelength is 1000nm.
Answers
Answer:
The energy of each transition is approximately \(1.98\times 10^{-19}\; \rm J\).
The frequency of photons released in such transitions is approximately \(3.00\times 10^{14}\; \rm Hz\).
Explanation:
The Rydberg Equation gives the wavelength (in vacuum) of photons released when the electron of a hydrogen atom transitions from one main energy level to a lower one.
Let \(\lambda_\text{vac}\) denote the wavelength of the photon released when measured in vacuum.Let \(R_\text{H}\) denote the Rydberg constant for hydrogen. \(R_\text{H} \approx 1.09678 \times 10^{7}\; \rm m^{-1}\).Let \(n_1\) and \(n_2\) denote the principal quantum number of the initial and final main energy level of that electron. (Both \(n_1\!\) and \(n_2\!\) should be positive integers; \(n_1 > n_2\).)The Rydberg Equation gives the following relation:
\(\displaystyle \frac{1}{\lambda_\text{vac}} = R_\text{H} \cdot \left(\frac{1}{{n_2}^2}} -\frac{1}{{n_1}^2}\right)\).
Rearrange to obtain and expression for \(\lambda_\text{vac}\):
\(\displaystyle \lambda_\text{vac} = \frac{1}{\displaystyle R_\text{H}\cdot \left(\frac{1}{{n_2}^2} - \frac{1}{{n_1}^2}\right)}\).
In this question, \(n_1 = 7\) while \(n_2 = 3\). Therefore:
\(\begin{aligned} \lambda_\text{vac} &= \frac{1}{\displaystyle R_\text{H}\cdot \left(\frac{1}{{n_2}^2} - \frac{1}{{n_1}^2}\right)} \\ &\approx \frac{1}{\displaystyle 1.09678 \times 10^{7}\; \rm m^{-1} \cdot \left(\frac{1}{3^2} - \frac{1}{7^2}\right)} \approx 1.0 \times 10^{-6}\; \rm m \end{aligned}\).
Note, that \(1.0\times 10^{-6}\; \rm m\) is equivalent to \(1000\; \rm nm\). That is: \(1.0\times 10^{-6}\; \rm m = 1000\; \rm nm\).
Look up the speed of light in vacuum: \(c \approx 3.00\times 10^{8}\; \rm m \cdot s^{-1}\). Calculate the frequency of this photon:
\(\begin{aligned} f &= \frac{c}{\lambda_\text{vac}} \\ &\approx \frac{3.00\times 10^{8}\; \rm m\cdot s^{-1}}{1.0\times 10^{-6}\; \rm m} \approx 3.00 \times 10^{14}\; \rm Hz\end{aligned}\).
Let \(h\) represent Planck constant. The energy of a photon of wavelength \(f\) would be \(E = h \cdot f\).
Look up the Planck constant: \(h \approx 6.62607 \times 10^{-34}\; \rm J \cdot s\). With a frequency of \(3.00\times 10^{14}\; \rm Hz\) (\(1\; \rm Hz = 1\; \rm s^{-1}\),) the energy of each photon released in this transition would be:
\(\begin{aligned}E &= h \cdot f \\ &\approx 6.62607 \times 10^{-34}\; \rm J\cdot s^{-1} \times 3.00 \times 10^{14}\; \rm s^{-1} \\ &\approx 1.98 \times 10^{-19}\; \rm J\end{aligned}\).
The energy of the transition between n = 7 and n = 3 is 1.96 × 10^-19 J while the frequency is 3 × 10^14 Hz.
Using the Rydberg Equation for energy;
ΔE = -RH(1/n^2final - 1/n^2initial)
Given that;
nfinal = 3
ninitial = 7
RH = 2.18 × 10^-18 J
ΔE = - 2.18 × 10^-18(1/3^2 - 1/7^2)
ΔE = - 2.18 × 10^-18(0.11 - 0.02)
ΔE = - 1.96 × 10^-19 J
For the second part;
Since the wavelength is 1000nm, we have;
λ = 1000nm
c = 3 × 10^8 m/s
f = ?
c = λf
f = c/λ
f = 3 × 10^8 m/s/1000 × 10^-9 m
f = 3 × 10^8 m/s/ 1 × 10^-6 m
f = 3 × 10^14 Hz
Learn more: https://brainly.com/question/18415575
1.5 L of a 0.77 M KCl solution find moles of KCl
Answers
To find the number of moles of a given solution, multiply the volume of the solution (in liters) by the molarity of the solution (in molarity). In this case, the volume of the solution is 1.5 liters and the molarity is 0.77 molarity.
What is the molarity ?Molarity is a measure of concentration of a solute in a solution expressed as the number of moles of solute per liter of solution (mol/L). It is a commonly used unit of concentration in chemistry. Molarity is often used to measure the amount of a solute in a given volume of solution and is also used to calculate the amount of a solute needed to create a given volume of solution at a given concentration.
Moles of KCl = 1.5 L x 0.77 M = 1.155
To learn more about molarity
https://brainly.com/question/26528084
#SPJ1
state thermodyNamic
Answers
Answer:
thermodynamic deals with the heat and temperature and their relationship with energy
Explanation:
i hope this will help you
Answer: I Hope It Helps :)
The First Law of Thermodynamics (Conservation) states that energy is always conserved, it cannot be created or destroyed. In essence, energy can be converted from one form into another.The third law of thermodynamics states that the entropy of a system at absolute zero is a well-defined constant. The Second Law of Thermodynamics says that processes that involve the transfer or conversion of heat energy are irreversibleThe fourth law of thermodynamics is the conservation of timeExplanation:
a. Identify the structures shown in the diagram. b. Identify the information that is contained within these structures. c. Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person. d. Explain why the structures are in pairs.
Answers
The answer responses to the structures shown in the diagram are:
A. chromosomes
C. They would be the same.
B. They are in pairs because each one comes from a different parent.
What is the structure about?The chromosomes are in pairs because humans have a diploid number of chromosomes, meaning they have two sets of chromosomes, one inherited from each parent.
The nucleus is important in eukaryotic cells and has many important parts that help the cell work properly. There are some parts inside cells called the nuclear membrane, nucleoplasm, nucleolus, and chromatin. Chromatin is made up of DNA and other proteins.
Every part of a person's body has the same genes, but the way they are organized can be different in different types of cells. The chromosomes in our skin cells might not be the same as the chromosomes in our muscle cells, even if they come from the same person.
Learn more about nucleus from
https://brainly.com/question/9376695
#SPJ1
Identify the structures shown.
A. chromosomes
B. mitochondria
C. nuclei
D. vacuoles
C
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Describe how the structures from this cell would compare to the structures in the nucleus of another body cell from the same person.
A. There would be longer.
B. They would be shorter.
C. They would be the same.
D. They would be different.
Explain why the structures are in pairs.
A. They aren't in pairs.
B. They are in pairs because each one comes from a different parent.
C. This cell is making a copy of itself.
D. The cell always has 2 copies in case 1 is damaged.
What type of energy drives the turbines that produce electricity from water?
Answers
Answer:
Wind energy
Explanation:
Answer: Wind energy
Explanation:
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
Using the tables, what is the standard entropy change for the following reaction?
CH4 (8) + 2 02 (8) - CO2 (g) + 2 H20 (g)
Report your answer in units of J/K, but do not type the units.
Answers
The standard entropy change for the given reaction is -319.1 J/K.
The standard entropy change (ΔS°) for a reaction can be determined by calculating the difference between the sum of the standard entropies of the products and the sum of the standard entropies of the reactants.
In this reaction, the reactants are CH4 (methane) and O2 (oxygen gas), and the products are CO2 (carbon dioxide gas) and H2O (water vapor).
Using the standard entropy values from the tables, the sum of the standard entropies of the reactants is 186.3 J/K, and the sum of the standard entropies of the products is 505.4 J/K.
ΔS° = (ΣS° products) - (ΣS° reactants)
= 505.4 J/K - 186.3 J/K
= -319.1 J/K
Therefore, the standard entropy change for this reaction is -319.1 J/K. A negative value indicates that the reaction results in a decrease in entropy, meaning the products have less disorder or randomness compared to the reactants.
for such more questions on entropy
https://brainly.com/question/419265
#SPJ8
Mendeleev originally organized his periodic table by _________, whereas now we arrange it by ____________.
atomic mass, atomic number
size, atomic mass
alphabetically, atomic number
atomic number, atomic mass
Answers
Answer: atomic mass, atomic number
Explanation:
Question 2
Which of the following models the arrangement of atoms in a gas?
O None of these
PLEASE HELP ASAP
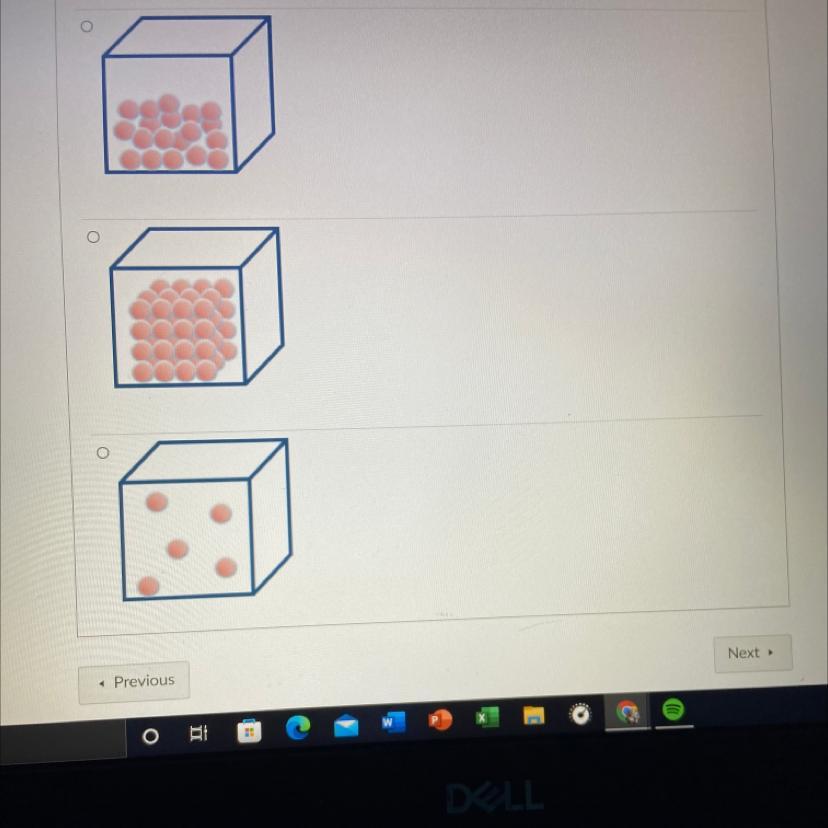
Answers
Answer:
The option where the atoms are furthest apart.
Explanation:
Gasses are the most energetic of the three basic states of matter, their atoms have more kinetic energy than either solids or liquids and will therefore also have the most spread out atoms.
The law that states when different compounds are formed by a combination of the same elements, different masses of one element combine with the same fixed to other elment
Answers
Answer:
The law of multiple proportions states that whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.
A binary molecule consists of how many atoms?
O1
02
3
4
Answers
Answer:
2
Explanation:
BIonary bi means 2
i hope this helps :)
What is the mass percent of sucrose (C12H22O11, Mm = 342 g/mol) in a 0.329-m sucrose solution?
Answers
Answer:
\(\% m/m=10.1\%\)
Explanation:
Hello,
In this case given the molal solution of sucrose, we can assume there are 0.329 moles of sucrose in 1 kg of solvent, thus, computing both the mass of sucrose and solvent in grams, we obtain:
\(m_{sucrose}=0.329mol*\frac{342g}{1mol}=112.5g\)
\(m_{solvent}=1000g\)
In such a way, we proceed to the calculation of the mass percent as follows:
\(\% m/m=\frac{112.5g}{112.5g+1000g}*100\%\\ \\\% m/m=10.1\%\)
Regards.
Which type of energy refers to the sum of potential and kinetic energies in the particles of a substance?
motion
stored
internal
heat
If you answer fast u get all the hearts
Answers
Explanation:
the sum of PE and KE is mechanical energy this means energy during motion and position I think the answer is motion and stored or it may be internal
Answer:
a
Explanation:
i did the test
which of the indicated protons in the following compound would appear most upfield in the 1h nmr spectrum?
Answers
P1 proton in the compound appear most upfield in the 1H NMR spectrum.
What is the main principle of NMR?
The foundation of NMR is the idea that all nuclei are electrically charged and that many of them have spins. Energy can go from the base energy to a higher energy level if an external magnetic field is provided.
The protons are most shielded ones among all the four types of protons as they experience +I effect from adjacent CH2 groups and are also situated far away from carbonyl group. therefore the -I effect is very less compared to the protons 2, 3 and 4. all these factors makes the proton 1 the most shielded and hence they will have very low delta value that is upfield.
Therefore, P1 proton in the compound appear most upfield in the 1H NMR spectrum.
To learn more about NMR spectroscopy from the given link.
https://brainly.com/question/21024524
#SPJ4
QUESTION 6 Calculate the molality of the HCI (aq) using the below information: Formula weight (g/mol): 36.465 Density of the solution (g/ml): 1.19 Weight %: 37.2 Molarity: 12.1 Your answer should have 3 sf) QUESTION 7 Calculate the molality of the NH3(aq)
Answers
A 12.1 M HCl solution with a density of 1.19 g/mL has a molality of 16.1 m.
What is molality?Molality is defined as the total moles of a solute contained in a kilogram of a solvent.
Let's suppose we have 1 L of the solution.
Step 1: Calculate the moles and mass of HCl (solute) in 1 L of solution.The solution is 12.1 M, that is, there are 12.1 moles of HCl in 1 L of solution.
The molar mass of HCl is 36.465 g/mol.
12.1 mol × 36.465 g/mol = 441 g
Step 2: Calculate the mass corresponding to 1 L of solution.The density of the solution is 1.19 g/mL.
1000 mL × 1.19 g/mL = 1190 g
Step 3: Calculate the mass of water (solvent) in 1190 g of solution.In 1190 g of solution, there are 441 g of HCl.
mWater = 1190 g - 441 g = 749 g = 0.749 kg
Step 4: Calculate the molality (b) of the solution.b = 12.1 mol / 0.749 kg = 16.1 m
A 12.1 M HCl solution with a density of 1.19 g/mL has a molality of 16.1 m.
Learn more about molality here: https://brainly.com/question/14623340
What is the molarity of a 9.0 L solution that contains 0.500 mol HCl?
A.8.29 M
B.0.879 M
C.0.018 M
D.0.056 M
Answers
Answer: 0.056
Explanation:
The molarity of the solution is the ratio of the number moles of solute to the volume of solution. Hence, the molarity of the given solution is 0.056 M. Thus, option D is correct.
What is molarity ?Molarity is a common term used to express the concentration of a solution . It is the ratio of the number of moles of solute to the volume of solution in liters.
Given that,
volume of HCl solution = 9 L
no. of moles of HCl = 0.50 moles.
Then,
molarity = no.of moles/volume in L
M = 0.50 Moles/9 L
= 0.056 M.
Therefore, the molarity of the solution is 0.056 M.
Find more on molarity :
https://brainly.com/question/16727614
#SPJ3
Which of the following technique is used to purify the impurities that are not very different in chemical properties of element? [a] Gas chromatography [b] Column chromatography [c] TLC [d] HPLC
Answers
Answer:
Explanation: Liquid Chromatography
I'm sorry if i'm wrong
MULTIPLE CHOICE QUESTION
How many grams of Al203 were
decomposed?
287.13
200g
888g
1148.52g

Answers
287.13 grams of Al₂O₃ were decomposed. Therefore, option A is correct.
What is molar mass ?The term molar mass is defined as the mass in grams of one mole of the compound. A mole of any substance is 6.022 × 10²³ molecules. It is called as Avogadro's number. The molar mass is a bulk property, it is not molecular, property of a substance.
2Al₂O₃ ⇒ 4Al + 3O₂
Thus, 4 mol of Al combine with 3 mol of oxygen to form 2 mol of Al₂O₃.
2 mol of Al corresponds to 2 × 27 = 54g
Thus, the weight of Al used in the reaction is 108 g.
Molar mass of Al₂O₃ is 101.96g/mol.
5.63 moles of Al = 5.63 × 54 / 101.96
= 287.13 grams
Thus, 287.13 grams of Al₂0₃ were decomposed, option A is correct.
To learn more about the molar mass, follow the link;
https://brainly.com/question/12127540
#SPJ1
The Thermal Energy
The thermal energy of an object is the total kinetic energy of its particles. An object's
thermal energy depends on the mass of the object, its temperature, its state of matter,
and its chemical composition. Larger objects have more thermal energy than smaller
objects of the same material and density at the same temperature. A liquid substance
has more thermal energy than the same mass of the substance in its solid form.
9. Suppose you have two identical objects made of the same mass of the same material.
If one object is 20 °C warmer than the other, which object has more thermal energy?
Answers
Thermal Energy is directly proportional to the temperature
Or
\(\\ \sf\longmapsto Thermal\: Energy\propto Temperature \)
So if temperature increases the thermal Energy also increases.
An object which is 20°C warmer than other has more thermal energy
Let's find the temperature when the pressure is equal to zero.
There are two ways of doing this, what are they? (equation line and what else?)
What is the temperature when the pressure equals zero? What is the significance of that number?
Answers
The significance of the point where the pressure drops to zero is that at that point the gases would completely show ideal behavior.
What is the gas law?The gas laws can be used to describe the behavior of the ideal gases. It is pertinent to note that the ideal gas law can strictly be applied to gases that are at a high temperature and low pressure.
We can be able to find the temperature when the pressure is equal to zero either be the use of the equation line or by experiment. In that case, we would be able to obtain the point at which the pressure drops to zero.
The significance of the point where the pressure drops to zero is that at that point the gases would completely show ideal behavior.
Learn more about idea gas:https://brainly.com/question/4194158
#SPJ1
The molar mass of Be is 9.01 g/mol.
How many atoms are in 18.02 g of Be?
A. 2.697 x 10-²² atoms Be
B. 162.4 atoms Be
C. 1.085 x 10²5 atoms Be
D. 1.204 x 1024 atoms Be
Help
Skip
Answers
The molar mass of Be is 9.01 g/mol. Atoms in 18.02 g of Be is : D.) 1.204 x 1024 atoms Be
What is molar mass?The mass in grams of one mole of the compound is known as molar mass of a substance.
As moles of Be = mass of Be / molar mass of Be
moles of Be = 18.02 g / 9.01 g/mol
moles of Be = 2 mol
number of atoms of Be = moles of Be x Avogadro's number
number of atoms of Be = 2 mol x 6.022 x 10²³
So, number of atoms of Be = 1.204 x 10²⁴
Therefore, the answer is option D, 1.204 x 10²⁴ atoms Be.
To know more about molar mass, refer
https://brainly.com/question/837939
#SPJ1
Question 8
A certain cation has five atoms and an overall charge of +1. Which of the following combinations of
formal charges on the five atoms is indicative of the "best" Lewis structure?
Answers
Answer:
0, 0, 0, +1, 0
Explanation:
Because the compound has a +1 charge overall, the sum of the formal charges must equal +1. The correct answer is the Lewis structure with as many atoms as possible having the formal charge of 0 with just a single atoms having the +1 formal charge.