the second-order rate constant for the decomposition of clo is 6.33×109 m–1s–1 at a particular temperature. determine the half-life of clo when its initial concentration is 1.61×10-8 m .
Answers
Given, The second-order rate constant for the decomposition of ClO is k = 6.33 x 109 M–1s–1Initial concentration of ClO is [ClO]₀ = 1.61 x 10⁻⁸ M.
To find the half-life of ClO, we can use the second-order integrated rate equation which is given by:1/ [A]t = 1/ [A]₀ + kt/2Where k is the rate constant and [A]₀ is the initial concentration of the reactant.Arranging the equation in terms of t gives: t1/2 = 1/k[A].
If we substitute the given values in the equation, we get:t1/2 = 1 Therefore, the half-life of ClO when its initial concentration is 1.61 x 10⁻⁸ M is 4.29 x 10⁻⁴ s.
To know more about decomposition visit :
https://brainly.com/question/14843689
#SPJ11
Related Questions
A 20.0 mL solution of NaOH is neutralized with 24.1 mL of 0.200 M HBr. What is the concentration of the original NaOH solution
Answers
Answer:
0.241 M
Explanation:
We'll begin by writing the balanced equation for the reaction. This is given below:
HBr + NaOH —> NaBr + H₂O
From the balanced equation above,
The mole ratio of acid, HBr (nₐ) = 1
The mole ratio of base, NaOH (n₆) = 1
Finally, we shall determine the concentration of the NaOH solution. This can be obtained as follow:
Volume of base, NaOH (V₆) = 20 mL
Volume of acid, HBr (Vₐ) = 24.1 mL
Concentration of acid, HBr (Cₐ) = 0.2 M
Concentration of base, NaOH (C₆) =?
CₐVₐ / C₆V₆ = nₐ/n₆
0.2 × 24.1 / C₆ × 20 = 1/1
4.82 / C₆ × 20 = 1
Cross multiply
C₆ × 20 = 4.82
Divide both side by 20
C₆ = 4.82 / 20
C₆ = 0.241 M
Therefore, the concentration of the NaOH solution is 0.241 M
Steel is an example of a pure substance because it is made up of two different metals melted together. (4 points) True False
Answers
Answer:
false
Explanation:
8. A rock has a density of 4 g/ml and a mass of 16 grams. What is the volume this rock occupies?
Answers
Answer:
The answer is
4.0 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \\ \)
From the question
mass of rock = 16 g
density = 4 g/mL
The volume of the rock is
\(volume = \frac{16}{4} \\ \)
We have the final answer as
4.0 mLHope this helps you
Jeff is picking up a 140N rock with a force of 85N if Chris comes to help and Chris can lift with a force of 60N what's the net force and direction of motion
Answers
Answer:
well there combined net force is 145N so the rock will go up and they will have 5 N to spare
Explanation:
A rod of iron of uniform density has a thickness such that a one-inch-long segment of it weighs 3. 88 ounces. Given that there are 0. 035273 ounces in a gram and 2. 54 centimeters in an inch, how many grams would a 14. 79 cm length of the same iron rod weigh? a. 73. 30 g b. 274. 49 g c. 640. 51 g d. 1,626. 89 g.
Answers
The number of grams of iron present in 14.79 cm length of the same iron rod is 640.51 g.
What is ounces?Ounces is a unit of mass which is used to define a very little amount of anything.
In the question, given that:
In one inch of iron rod whose weighs is 3.88 ounces = 2.54 cm long
In 1 g = 0.035273 ounces present
So we can calcuylate the number of grams in which 3.88 ounces present as:
x = (3.88 ounces) / (0.035273 ounces/g)
x = 110g
So, in 110g = 3.88 ounces present
And in 2.54 cm = 110g of iron present
In 14.79 cm, present grams of iron can be calculated as:
y = (14.79cm x 110g) / 2.54cm
y = 640.51 g
Hence option (c) is correct i.e. 640.51 g.
To know more about ounces, visit the below link:
https://brainly.com/question/24505595
The weight of the iron rod with 14.79 cm length with same density is given by: Option C; 640.51 g
How are density, volume and mass of a substance related?Suppose that a finite amount of substance is there having its properties as:
mass of substance = m kgdensity of substance = d kg/m³volume of that substance = v m³Then, they are related as:
\(d = \dfrac{m}{v}\)
We know that:
1 ounce = 28.3495 g approxNow, from the facts given in the problem, we have:
The weight of first iron rod which is one inches long = 3.88 ounces
The new rod is 14.79 cm long. And 1 cm = 0.3937 inches approx, thus the new rod is: \(14.79 \times 0.3937 \approx 5.8228 \: \rm inches\)
As for 1 inch long rod, there is weight of 3.88 ounces, thus:
for 5.8228 inch rod, the weight of the rod would be \(5.8228 \times 3.88 \approx 22.5924 \: \rm ounces\)
And since 1 ounce = 28.3495 g approx
Thus, weight of the new rod in grams would be \(22.5924\times 28.3495 \approx 640.485 \approx 640.51\: \rm g\)
Thus, the weight of the iron rod with 14.79 cm length with same density is given by: Option C; 640.51 g
Learn more about density here:
https://brainly.com/question/952755
I can't leave till I answer please help!!

Answers
Answer:
The answer is 2 all you do is divide the 6.02 and 3.01 and you get 2
which of the following incorrectly describes mechanisms of co2 transport? which of the following incorrectly describes mechanisms of co2 transport? just over 20% of co2 is carried in the form of carbaminohemoglobin attached to the heme part of hemoglobin 7-10% of co2 is dissolved directly into the plasma as bicarbonate ions in plasma
Answers
The following statement that incorrectly describes mechanisms of CO2 transport is "7-10% of CO2 is dissolved directly into the plasma as bicarbonate ions in plasma."
The transport of carbon dioxide in the blood is done in three ways which are:
Dissolved CO2 Carbaminohaemoglobin Bicarbonate ions in the plasma
Dissolved CO2: This is the smallest fraction of carbon dioxide carried by the blood. Dissolved CO2 combines with water in the blood to form carbonic acid (H2CO3). This reaction is facilitated by an enzyme known as carbonic anhydrase. Carbonic acid dissociates to form H+ and bicarbonate ions (HCO3-).
Carbaminohaemoglobin: Just like oxygen, carbon dioxide can also bind with hemoglobin. Carbon dioxide binds with hemoglobin to form carbaminohaemoglobin. Carbaminohaemoglobin accounts for about 20% of the carbon dioxide transported by the blood.
Bicarbonate ions in the plasma: This is the largest fraction of carbon dioxide transported by the blood. Carbon dioxide diffuses from the tissues into the blood where it combines with water to form carbonic acid. Carbonic acid dissociates to form H+ and bicarbonate ions (HCO3-). The bicarbonate ions diffuse into the plasma where they are transported to the lungs. In the lungs, the bicarbonate ions diffuse back into the red blood cells. The bicarbonate ions are converted back to carbon dioxide, which is then exhaled.
The statement that incorrectly describes mechanisms of CO2 transport is "7-10% of CO2 is dissolved directly into the plasma as bicarbonate ions in plasma." This is incorrect because about 70% of CO2 is transported as bicarbonate ions, not 7-10%. Therefore, the statement is false.
For more question on bicarbonate ions click on
https://brainly.com/question/29315144
#SPJ11
The mistake in the question's descriptions concerning CO2 transport involves the part of hemoglobin that carbaminohemoglobin binds to (it's the globin part, not the heme part), and the proportion of CO2 carried as bicarbonate ions in the plasma (it's about 70%, not 7-10%).
Explanation:The mechanisms described here for the transport of CO2 in the body are partially incorrect. In the human body, around 20% of CO2 is indeed transported as carbaminohemoglobin, but it binds to the globin part, not the heme part, of the hemoglobin protein. Additionally, the primary way that CO2 is transported is as bicarbonate ions, but these bicarbonate ions constitute around 70%, not 7-10%, of the CO2 carried in the plasma. Therefore, the incorrect descriptions mentioned in the question are that carbaminohemoglobin binds to the heme part of hemoglobin and that only 7-10% of CO2 is carried as bicarbonate ions in the plasma.
Learn more about CO2 transporthttp://brainly.com/question/31063166
Particulate Electrical Forces Unit Test for Connexus, Chemistry A
I've already finished the questions, but im stuck on the written parts 16-18, can anyone give a hand? :(
Answers
I’m sorry no one helped you with those questions. But could you please be a doll and give me the answer for the multiple-choice questions 1-15. Pleaseee I’m so desperate.?
valence electrons are electrons located group of answer choices in the innermost energy level of an atom. in the outermost energy level of an atom. in the nucleus of an atom. in the first three shells of an atom. throughout the atom.
Answers
Valence electrons are described as the electrons that are located in the outermost shell of the atom.
Valence electrons are defined as the electrons which occupy the outermost shell or highest energy level of an atom while core electrons are those occupying the innermost shell or lowest energy levels.
Let's consider an example where, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. You can write the configuration of oxygen's valence electrons as 2s²2p⁴. Generally, a single electron or one of two or more electrons in the outer shell of an atom that is responsible for the chemical properties of the atom.
Learn more about valence electrons from the link given below.
https://brainly.com/question/2725863
#SPJ4
Match each type of mass movement to its description.
the rapid downhill flow of a mixture of rock,
soil, and water
slump
mudflow
the slow downhill movement of rock and soil
creep
the rapid downhill movement of a mass of
rock, debris, or soil
landslide
a loosely connected mass of rock and soil
that moves a short distance

Answers
Answer: np
Explanation:

Correctly matched are :
landside - rapid movement of rock, debris, and soil. mudflow - mixture of rocks, soil, water. slump - loosely mass of rocks and soil.What is a landslide?A landslide is also called a land slip. It refers to several forms of mass wasting and may include a wide range of ground movements. They occur in a variety of environments characterize by gentle and steep slope gradients.
The mudflow is a mixture of rocks and soil along with water. While a creep is a slow downloads movement of rocks and soil.
Find out more information about the mass movement.
brainly.com/question/6068732
nuclei such as protons do not fuse at low temperatures because their speeds are not enough to overcome their
Answers
Answer:
So what is the question ..
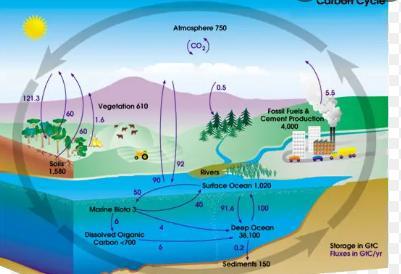
The combination of fossil fuel use and deforestation has emitted approximately 480 gigatons of carbon over the last century, but the amount of carbon in the atmosphere has only increased by approximately 190 gigatons. About 110 gigatons of this missing carbon went into which reservoir of the carbon cycle?.
Answers
About 110 gigatons of this missing carbon went into water reservoir of the carbon cycle.
What is carbon cycle?The biogeochemical cycle in which carbon is exchanged between the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth is known as the carbon cycle.
According to the question, emitted amount of carbon is 480 gigatons but the amount of carbon in the environment is approx 190 gigatons, so the remaining amount of the carbon was stoored by the oceans and other water reservoir, which may be get clear throug the attached diagram.
Hence remaining gigatons of carbon went into water reservoir.
To know more about carbon cycle, visit the below link:
https://brainly.com/question/24293689
#SPJ1
A reaction between 1. 7 moles of zinc iodide and excess sodium
Answers
The percent yield of zinc carbonate is 5.91%. This suggests that the reaction did not go to completion, and there was likely some loss of product during the experiment.
To find the percent yield of zinc carbonate, we need to compare the actual yield (what was obtained in the experiment) to the theoretical yield (what would be obtained if the reaction went to completion).
First, let's calculate the theoretical yield of zinc carbonate:
From the balanced equation, we can see that 1 mole of ZnI2 reacts with 1 mole of \(Na_{2}CO_{3}\) to produce 1 mole of \(ZnCO_{3}\).Since we have 1.7 moles of ZnI2, we would need 1.7 moles of \(Na_{2}CO_{3}\) to react completely.The molar mass of \(ZnCO_{3}\) is 125.39 g/mol, so the theoretical yield of \(ZnCO_{3}\) would be:theoretical yield = 1.7 mol ZnCO3 x 125.39 g/mol = 213.07 gNow, let's calculate the percent yield:
The actual yield \(ZnCO_{3}\) is given as 12.6 g.
The percent yield is calculated as:
percent yield = (actual yield / theoretical yield) x 100%percent yield = (12.6 g / 213.07 g) x 100% = 5.91%Learn more about zinc carbonate
https://brainly.com/question/31114294
#SPJ4
Full Question: A reaction between 1.7 moles of zinc iodide and excess sodium carbonate yields 12.6 grams of zinc carbonate. This is the equation for the reaction: Na2CO3 + ZnI2 → 2NaI + ZnCO3. What is the percent yield of zinc carbonate? The percent yield of zinc carbonate is %
Which of the following has the highest concentration?
A 1.2 M phosphoric acid
B 0.9 M phosphoric acid
C 3 M phosphoric acid
D 0.17 M phosphoric acid
Answers
The higher the molarity, the more concentrated the solution is.
if you use too much hot solvent when dissolving your crude compound, how will that impact the recovery of your compound and why?
Answers
Recrystalization will occur.The solution may become too diluted for crystals to form if you add too much solvent. Impurities will be captured by a hastily formed crystal's lattice. The crystals that result will also be smaller.
What is Recrystalization?Recrystallization is a physical process used to separate compounds based on how soluble they are. Heating the material to dissolve the compound with impurities in a mixture of a suitable solvent completes the procedure. We can remove the desired chemical or contaminants from the mixture using this method.
The solution may become too diluted for crystals to form if you add too much solvent. The flask needs to be gently cooled, first at room temperature and then in cold water. Impurities will be captured by a hastily formed crystal's lattice. The resulting crystals will also be smaller.
This method is used to harden steel in order to eliminate all strain hardening side effects, including the significant plastic deformation brought on by cold working.The crystals that frequently form when the compound precipitates out gave it its name. The natural expansion of larger ice crystals at the expense of smaller ones is another definition of recrystallization.Some commonly effective mixes include diethyl ether-methanol (or ethanol) for polar molecules (particularly esters, alcohols, and hydrocarbons) and diethyl ether-petroleum ether (or benzene) for strongly linked solids (notably amides, alcohols), as well as many natural products.The three main types of recrystallization are;
Single-solvent recrystallization.Multi-solvent recrystallization.Hot filtration-recrystallization.To know more about Recrystalization, refer to:
https://brainly.com/question/10194206
#SPJ1
Which orbital notation represents the second principal energy level of a silicon atom in the ground state?
Answers
Answer:
2
Explanation:
Silicon is one the elements found in nature and well positioned the periodic table.
It has an atomic number of 14 with an electronic configuration:
1s² 2s² 2p⁶ 3s² 3p⁴
From this formula the orbital notation that represents the second principal energy level is 2.
The main energy level in which the orbital is located or the average distance of the orbital from the nucleus is the principal quantum number.
It takes the number 1,2,3,4,5 e.t.c
The solubility of argon in water at 25 degree C is 0.150 mol/L. What is the Henry's Law constant for argon if the partial pressure of argon in air is 0.0984 atm? a. 1.52 mole/L middot atm b. 0.0164 mole/L middot atm c. 12.5 mole/L middot atm d. 0.656 mole/L middot atm e. 0.0801 mole/L middot atm
Answers
The Henry's Law constant for argon if the partial pressure of argon in air is 0.0984 atm is 1.52 mole/L *atm.
Henry's regulation states that at a regular temperature, the amount of a given fuel that dissolves in a liquid is immediately proportional to the partial pressure of that gas in equilibrium with that liquid. Henry's regulation states that the solubility of a fuel in a liquid is immediately proportional to the pressure of the gas.
Pressure of argon = p = 0.0984 atm
Molar solubility at 25°C, S = 0.150 mol/l
Calculating the Henry's Law constant,
S =KP
K =S/P
0.150/0.0984
= 1.52 mol/L atm
Hence the Henry's Law constant (K) = = 1.52 mol/L atm
Learn more about Henry's Law here:-https://brainly.com/question/23204201
#SPJ4
which empirical gas law describes the relationship between the volume and temperature of a gas when the number of moles and pressure are constant? please choose the correct answer from the following choices, and then select the submit answer button. answer choices
Answers
Charles's Law helps us understand the relationship between temperature and volume of a gas when other factors, such as pressure and number of moles, remain constant. The empirical gas law that describes the relationship between the volume and temperature of a gas when the number of moles and pressure are constant is Charles's Law.
This can be expressed mathematically as: V₁/T₁ = V₂/T₂
Where V₁ and V₂ represent the initial and final volumes of the gas, and T₁ and T₂ represent the initial and final temperatures of the gas, respectively. The relationship can also be stated as: V/T = constant
To illustrate this law, let's consider an example. Imagine a balloon filled with a fixed number of moles of gas at a constant pressure. If we were to heat the balloon by placing it in a warm environment, the temperature of the gas inside the balloon would increase.
To know more about empirical gas law visit:
brainly.com/question/31191366
#SPJ11
Someone help Match them

Answers
PLEASE ANSWER NOW!!! This chart shows what happens when each object is placed on a balance with a 10 kg weight on the other side. Which statement is best supported by the information in the chart? Object 1 is the heaviest object. Object 3 is heavier than Object 1. The weight is heavier than Object 2. The weight is the heaviest object.

Answers
Answer:
Balanced forces do not cause a change in motion. When balanced forces act on an object at rest, the object will not move. ... Forces that cause a change in the motion of an object are unbalanced forces. Unbalanced forces are not equal and opposite.
Explanation:
Any push or pull is a force. To describe a force, you must know two things. You must know the size of the force and the direction of the force. Suppose two teams are playing tug of war. Each team is pulling with equal force, but in opposite directions. Neither team can make the other team move. Forces that are equal in size but opposite in direction are called balanced forces.
tug of war balanced
Balanced forces do not cause a change in motion. When balanced forces act on an object at rest, the object will not move. If you push against a wall, the wall pushes back with an equal but opposite force. Neither you nor the wall will move. Forces that cause a change in the motion of an object are unbalanced forces.
tug of war unbalanced 1
Unbalanced forces are not equal and opposite. Suppose that one of the teams in tug of war pulls harder than the other team. The forces would no longer be equal. One team would be able to pull the other team in the direction of the larger force.
Force and Motion
More than one force can act on an object at the same time. If you hold a paper clip near a magnet, you, the magnet and gravity all exert forces on the paper clip. The combination of all the forces acting on an object is the net force. When more than one force is acting on an object, the net force determines the motion of the object. In this example, the paper clip is not moving, so the net force is zero.
How do forces combine to form the net force? If the forces are in the same direction, they add together to form the net force. Suppose you and a friend are asked to move a piano for the music teacher. To do this, you pull on one end of the piano, and your friend pushes on the other end. Together, your forces add up to enough force to move the piano. This is because your forces are in the same direction. Because the forces are in the same direction, they can be added together to determine the net force. In this case, the net force is 45 N, which is plenty to move a piano - if it is on wheels, that is!
net force piano
If two forces are in opposite directions, then the net force is the difference between the two forces, and it is in the direction of the larger force. Consider two dogs playing tug of war with a short piece of rope. Each is exerting a force, but in opposite directions.
Notice below that the dog on the left is pulling with a force of 10 N, and the dog on the right is pulling with a force of 12 N. Which dog do you think will win the tug of war? Because the forces are in opposite directions, the net force is determined by subtracting the smaller force from the larger one. In this case, the net force is 2 N in the direction of the dog on the right. Give that dog a dog biscuit!
Answer:
A. Object 1 is the heaviest
Hope it works!
Explanation:
Hey is plasma a state of matter
Yes or no
I will mark you brainliest
Answers
Answer:
no
Explanation:
Which group has the highest ionization energies? Explain why.
Answers
Answer:
nobel elements (gr. 18) because they are fully stable due to octet complete--------------------------------------------------
under standard conditions (298 k and 1 atm), which statement is true? refer to the constants for thermodynamic properties under standard conditions. diamond converts to graphite spontaneously graphite converts to diamond spontaneously none of the above how can the spontaneity of the reaction be reversed? increase the temperature decrease the temperature none of the above
Answers
Under standard conditions diamond converts to graphite spontaneously.
What are thermodynamic constants?The constant of thermodynamic equilibrium K is the correct quotient of species activities in reaction equilibrium. An activity cannot be many orders of magnitude greater than 1 at ordinary temperatures and pressures.Thermodynamic properties are defined as system characteristics that can specify the system's state. Some constants, such as R, do not explain the state of a system and so are not attributes.The transformation from diamond to graphite is spontaneous and advantageous, according to thermodynamics. However, because kinetics rather than thermodynamics governs this reaction, it proceeds at an exceedingly slow rate. Diamond is therefore kinetically stable but thermodynamically unstable.To learn more about Thermodynamics. refer,
https://brainly.com/question/13059309
#SPJ4
determine the molarity of a solution prepared by diluting 12.2 grams NaCl with enough water to make 771 mL of solution
Answers
we need to calculate the volume of the final solution after dilution. We know that we added enough water to make a total volume of 771 mL, so the volume of the NaCl solution must be?
what is moles?The mole is defined as exactly 6.02214076×1023 elementary entities. Depending on the nature of the substance, an elementary entity may be an atom, a molecule, an ion, an ion pair, or a subatomic particle such as a proton.
moles NaCl = mass / molar mass
moles NaCl = 12.2 g / 58.44 g/mol
moles NaCl = 0.209 moles
volume NaCl solution = total volume - volume of water added
volume NaCl solution = 771 mL - volume of water added
To calculate the volume of water added, we can use the fact that we diluted the solution. We can set up a ratio of the initial concentration (which is the same as the molarity) to the final concentration, and use this ratio to solve for the volume of water added:
initial concentration * initial volume = final concentration * final volume
0.209 moles / initial volume = final concentration / 771 mL
final concentration = 0.209 moles / initial volume * 771 mL
Since we diluted the solution, we know that the final concentration is less than the initial concentration. We also know that we added water, which means the final volume is greater than the initial volume. We can set up a new ratio using the dilution factor (the ratio of final volume to initial volume) to solve for the final concentration:
final concentration = initial concentration / dilution factor
final concentration = initial concentration / (final volume / initial volume)
final concentration = initial concentration * (initial volume / final volume)
Now we can substitute in our values and solve for the final concentration:
final concentration = 0.209 moles * (771 mL / volume NaCl solution)
Finally, we can substitute this expression for final concentration into our previous equation and solve for the volume of water added:
0.209 moles / initial volume * 771 mL = 0.209 moles * (771 mL / volume NaCl solution) * (initial volume / final volume)
Simplifying and rearranging:
volume NaCl solution = initial volume * (0.209 moles / final concentration)
volume NaCl solution = initial volume * (0.209 moles / (0.209 moles * (771 mL / volume NaCl solution) * (initial volume / final volume)))
volume NaCl solution = initial volume * (771 mL / (0.209 * final volume))
Now we can substitute in our values and solve for the volume of the NaCl solution:
771 mL - volume of water added = initial volume
771 mL - (initial volume * (771 mL / (0.209 * final volume))) = initial volume
771 mL / (0.209 * final volume) = 1 + (initial volume / final volume)
(771 mL / (0.209 * final volume)) - (initial volume / final volume) = 1
771 mL / (0.209 * final volume) - (771 mL - volume NaCl solution) / final volume = 1
Simplifying and rearranging:
final volume = volume NaCl solution / (1 - 0.209 * (771 mL / volume NaCl solution))
Now we can substitute in our values and solve for the final volume:
final volume = 771 mL / (1 + 0.209 * (771 mL / volume NaCl solution))
Finally, we can use the final volume to calculate the final concentration (which is the molarity):
final concentration = 0.209 moles * (initial volume / final volume)
final concentration = 0.209 moles * (771 mL / (771 mL / (1 + 0.209 * (771 mL / volume NaCl solution))))
final concentration = 0.209 moles / (1 + 0.209 * (771 mL / volume NaCl solution))
Therefore, the molarity of the solution prepared by diluting 12.2 grams of NaCl with enough water to make 771 mL of solution is approximately 0.544 M.
To determine the molarity of a solution prepared by diluting 12.2 grams of NaCl with enough water to make 771 mL of solution, follow these steps:
Step 1: Calculate the moles of NaCl
To do this, divide the mass of NaCl (12.2 grams) by its molar mass (58.44 g/mol for NaCl).
Moles of NaCl = 12.2 grams / 58.44 g/mol = 0.209 moles
Step 2: Convert the volume of the solution to liters
Since molarity is expressed in moles per liter, convert the volume from mL to L by dividing it by 1000.
Volume in liters = 771 mL / 1000 = 0.771 L
Step 3: Calculate the molarity
Divide the moles of NaCl by the volume of the solution in liters.
Molarity = 0.209 moles / 0.771 L = 0.271 M
So, the molarity of the solution is 0.271 M.
To know more about mole visit:
https://brainly.com/question/15209553
#SPJ11
2 sticks of butter, 1-teaspoon baking soda, 1.5 cups sugar, 2 teaspoons cream of tartar, 2 eggs, 0.5 teaspoon salt, 2.75 -cup flour, 4 teaspoons cinnamon-sugar. In your kitchen, you have 24 sticks of butter, 10.5 cups of sugar, 1.5 dozen eggs, 22 cups of flour, 15 teaspoons of baking soda, 45 teaspoons of cream of tartar, 50 teaspoons of salt, and 40 teaspoons of cinnamon sugar. How many batches of Snickerdoodles can you make? What is the limiting reactant?
Answers
The quantity of the limiting reagent determines how many batches of Snickerdoodles are produced. Four Snickerdoodles can be created since cinnamon-sugar has a lower reagent need.
When a chemical reaction is complete, the limiting reagent—also known as the limiting reactant and limiting agent—is the reactant that has been completely consumed. Since the reaction is unable to continue without this reagent, the quantity of product that may be produced is constrained. surplus reagents or surplus reactants are any reagents that are present in amounts greater than those necessary to cause a reaction with the limiting reagent. The quantity of the limiting reagent determines how many batches of Snickerdoodles are produced. Four Snickerdoodles can be created since cinnamon-sugar has a lower reagent need.
To know more about limiting reagent, here:
https://brainly.com/question/31171741
#SPJ1
A sample of Xe gas is observed to effuse through a pourous barrier in 4.83 minutes. Under the same conditions, the same number of moles of an unknown gas requires 2.29 minutes to effuse through the same barrier.
The molar mass of the unknown gas is
_____ g/mol.
Answers
Answer:
28.93 g/mol
Explanation:
This is an extension of Graham's Law of Effusion where \(\frac{R1}{R2} = \sqrt{\frac{M2}{M1} } = \frac{t2}{t1}\)
We're only talking about molar mass and time (t) here so we'll just concentrate on \(\sqrt{\frac{M2}{M1} } = \frac{t2}{t1}\). Notice how the molar mass and time are on the same position, recall effusion is when gas escapes from a container through a small hole. The time it takes it to leave depends on the molar mass. If the gas is heavy, like Xe, it would take a longer time (4.83 minutes). If it was light it would leave in less time, that gives us somewhat an idea what our element could be, we know that it's atleast an element before Xenon.
Let's plug everything in and solve for M2. I chose M2 to be the unknown here because it's easier to have it basically as a whole number already.
\(\sqrt{\frac{M2}{131} } = \frac{2.29}{4.83}\)
The square root is easier to deal with if you take it out in the first step, so let's remove it by squaring each side by 2, the opposite of square root essentially.
\((\sqrt{\frac{M2}{131} } )^2= (\frac{2.29}{4.83})^2\)
\({\frac{M2}{131} } = (0.47)^2\)
\({\frac{M2}{131} } = 0.22\)
M2= 0.22 x 131
M2= 28.93 g/mol
How are chemical reactions used in art
Answers
Answer:
Chemistry reactions are used in art for the following processes;
1) Analog photography
The photographic paper used in analog photography react when exposed to light such that the image on the film stains the photopaper
A series of chemicals are further used to develop the images now carried on the paper and water is used to rinse of the chemicals after the other chemical processes are complete
The photopaper, now bearing the developed photo is hung for it to be dried
2) Paint used for painting consists of several chemicals, including, minerals that serve as pigment, oils that serve as carrying agent, a thinner to prevent the paint from turning to solid
An artist therefore combines different chemicals for a given paint task
3) In the sculpting process
An original sculpture is produced by the artist with the aid of clay or plaster, from the original sculpture, on which wax coatings and chemicals are used to make a replica mold.
Copies of the sculpture can then be made by pouring material into the mold
Explanation:
THIS IS DUR IN LIKE 15 MINS PLS

Answers
Answer:
A. Wisconsin
Explanation:
In the graph, Wisconsin has the highest speed limit.
A change in matter that produces new substances is called a _____. 1.mixture 2.physical change 3.solution 4.chemical change
Answers
Answer:
Chemical Change
Explanation:
chemical bonds within the substance have been altered because a new substance has been produced. It cannot be a mixture because they do not involve changes in matter, it cannot be a physical change because they do not result in new substances, it is not a solution because those do not involve changes in matter.
Oxygen Gas (O₂)
A. 2 atoms of oxygen
B. 2 atoms of hydrogen
C. 3 atoms of oxygen
D. 1 atom of oxygen