The second atomic structure YES
No
Answers
Answer
The second part of the theory says all atoms of a given element are identical in mass and properties. The third part says compounds are combinations of two or more different types of atoms. The fourth part of the theory states that a chemical reaction is a rearrangement of atoms.
Explanation:
Related Questions
A student performed the heat of fusion lab and measured the heat of
fusion to be 313 J/g. Calculate the student's percent error. *
Answers
A student has performed the heat of fusion lab and measured the heat of fusion to be 313 J/g. In order to calculate the percent error, we need to know the experimental value and the true value of the heat of fusion.
The formula to calculate the percent error is as follows: Percent error = (experimental value - true value) / true value * 100%To calculate the percent error for the student who performed the heat of fusion lab and measured the heat of fusion to be 313 J/g, we need to know the true value of the heat of fusion. The true value of the heat of fusion of a substance is the amount of energy required to melt one gram of that substance without changing its temperature. This value can be found in a reference book or online database.
For example, the true value of the heat of fusion of water is 333.55 J/g at 0°C. Therefore, we can calculate the percent error as follows: Percent error = (313 J/g - 333.55 J/g) / 333.55 J/g * 100% = -6.17%The negative sign indicates that the student's measurement is lower than the true value. The percent error of -6.17% means that the student's measurement is 6.17% lower than the true value of the heat of fusion.
To know more about experimental visit:
https://brainly.com/question/5421090
#SPJ11
Valence electrons
Fill in the blanks
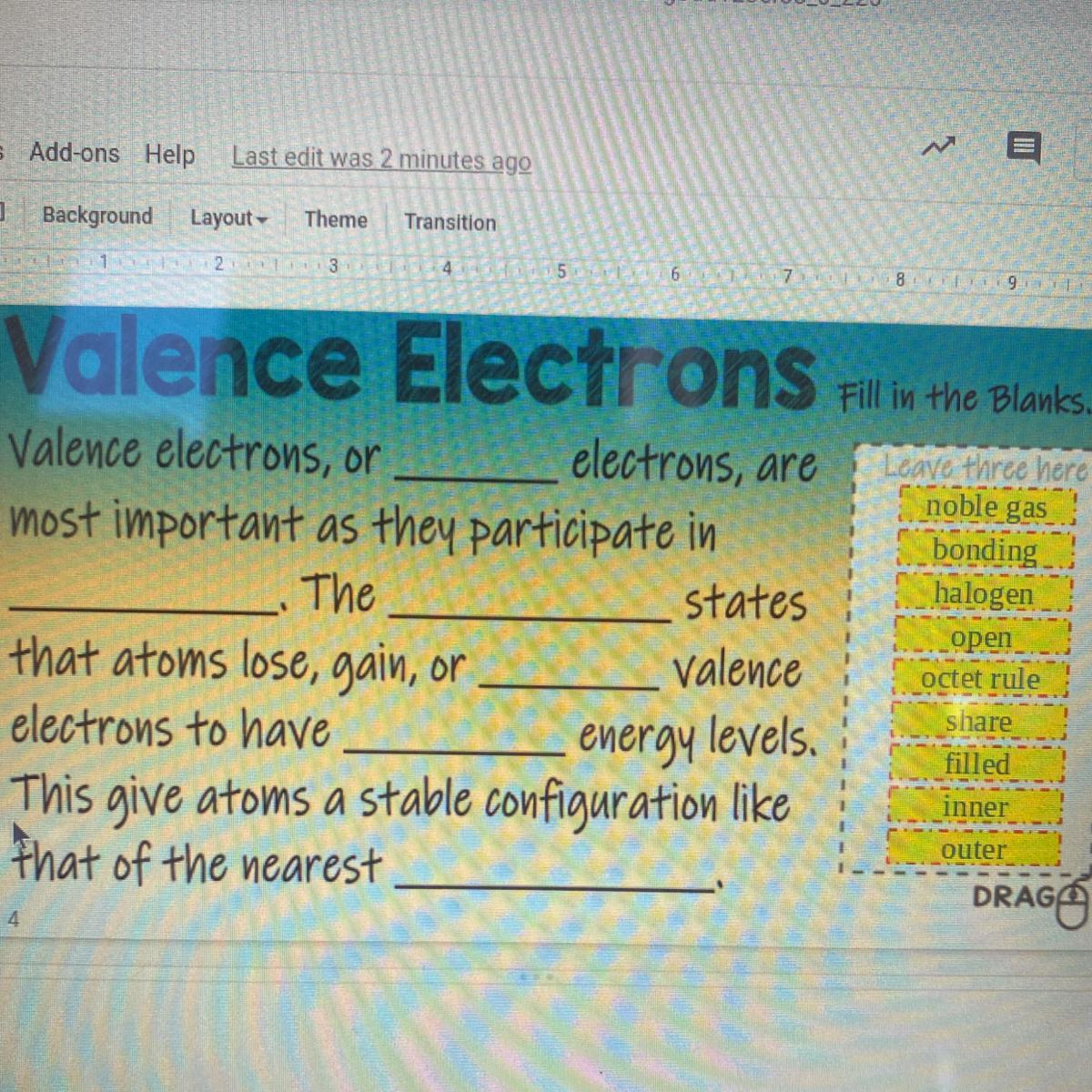
Answers
Answer:
Valence electrons or outer electrons are most important as they participate in bonding. The octet rule states that atoms gain, lose, or share valence electrons to have filled energy levels.. this gives atoms a stable configuration like that of the nearest noble gas.
Use the following information to answer numbers 20-22: Start with a 30M sucrose solution. Then you do a serial dilution by making 100 mL of a 1/10 dilution and repeat THREE more times. 20. Show the calculation for the first dilution. (1 pt) Answer: 21. Draw and label a diagram of the serial dilution with volumes and concentrations of the stock and dilution beakers. (2 pts) Answer: 22. Show your concentration calculations. (1 pt) Answer:
Answers
The concentrations for the second, third, and fourth dilutions would be 0.3M, 0.03M, and 0.003M, respectively.
In a serial dilution, a concentrated solution is successively diluted to obtain solutions with lower concentrations. In this case, starting with a 30M sucrose solution, a series of four 1/10 dilutions are performed. The first dilution involves making 100 mL of the 1/10 dilution. To answer question 20, the calculation for the first dilution needs to be shown. For question 21, a diagram of the serial dilution with labeled volumes and concentrations of the stock and dilution beakers needs to be drawn. Finally, for question 22, the concentration calculations for each dilution step need to be provided.
To calculate the first dilution, we need to determine the concentration of the resulting solution. Since it is a 1/10 dilution, the concentration would be 1/10 times the original concentration. Therefore, the concentration of the first dilution would be 30M / 10 = 3M.
For question 21, a diagram needs to be drawn to illustrate the serial dilution process. The diagram should include the stock solution with a volume of 30M, the first dilution beaker with a volume of 100 mL and a concentration of 3M, and labels indicating the volumes and concentrations at each step of the dilution process.
For question 22, the concentration calculations for each dilution step need to be provided. Starting with the first dilution at 3M, subsequent dilutions would be 1/10 of the previous concentration. Therefore, the concentrations for the second, third, and fourth dilutions would be 0.3M, 0.03M, and 0.003M, respectively.
Overall, these calculations and the diagram represent the process and concentrations involved in the serial dilution of the 30M sucrose solution.
To learn more about serial dilution click here: brainly.com/question/28548168
#SPJ11
Which type of bond is formed by glycogen synthase upon release of UDP?
A.α-1,4-Glycosidic bond
B.α-1,6-Glycosidic bond
C.β-1,4-Glycosidic bond
D.β-1,6-Glycosidic bond
Answers
Glycogen synthase forms an B)α-1,4-glycosidic bond upon release of UDP.
Glycogen synthase is an enzyme that catalyzes the formation of glycogen, a branched polysaccharide that serves as a storage form of glucose in animals. The enzyme adds glucose residues to the growing glycogen chain by forming α-1,4-glycosidic bonds between adjacent glucose molecules.
When the chain reaches a certain length, branching occurs through the formation of α-1,6-glycosidic bonds, catalyzed by the enzyme branching enzyme.
The release of UDP from glycogen synthase occurs after the addition of each glucose residue, and it is required for the enzyme to continue adding glucose residues to the growing glycogen chain. Therefore, glycogen synthase forms B) α-1,4-glycosidic bonds upon release of UDP.
For more questions like Enzymes click the link below:
https://brainly.com/question/31385011
#SPJ11
What kind of rocks might you expect to find on the Moon? Why do you think that?
Answers
Answer:
The Moon's surface is dominated by igneous rocks. The lunar highlands are formed of anorthosite, an igneous rock predominantly of calcium-rich plagioclase feldspar
Explanation:
which of the following formula/name pairs is incorrect? a. mnco3: manganese(ii) carbonate b. mgso4:magnesium sulfate c. n3o5: trinitrogen pentoxide d. bacl2: barium chloride e. fe2s3: iron(ii) sulfide
Answers
c. n3o5: tri nitrogen pentoxide is incorrect because the correct formula should be N2O5, representing two nitrogen atoms and five oxygen atoms in the compound.
The correct formula for trinitrogen pentoxide should be N2O5, not N3O5. Trinitrogen pentoxide consists of two nitrogen atoms (N2) and five oxygen atoms (O5). The prefix "tri-" indicates the presence of three nitrogen atoms. Therefore, the formula N2O5 correctly represents tri-nitrogen pentoxide.
Option c states N3O5 as the formula for tri-nitrogen pentoxide, which is incorrect because it suggests the presence of three nitrogen atoms and five oxygen atoms. The formula should have two nitrogen atoms and five oxygen atoms, as represented by N2O5.
The other formula/name pairs (a. MnCO3, b. MgSO4, d. BaCl2, and e. Fe2S3) are correct and match the correct names of the respective compounds (manganese(ii) carbonate, magnesium sulfate, barium chloride, and iron(ii) sulfide).
learn more about nitrogen atoms here:
https://brainly.com/question/33169732?
#SPJ11
The incorrect formula/name pair is Fe2S3: Iron(II) Sulphide. According to the formula Fe2S3, the correct name should be Iron(III) Sulphide.
Explanation:The question is asking to identify the incorrect formula/name pair among the given options. The pairs are: (a) MnCO3: Manganese(II) Carbonate, (b) MgSO4: Magnesium Sulfate, (c) N3O5: Trinitrogen Pentoxide, (d) BaCl2: Barium Chloride, and (e) Fe2S3: Iron(II) Sulphide.
Using the rules of naming chemical compounds, the incorrect pair is (e) Fe2S3: Iron(II) Sulphide. The Roman numeral (II) in 'Iron(II)' indicates the oxidation number of Iron. According to the given formula, Fe2S3, there are 2 atoms of Iron and 3 atoms of Sulfur. Hence, the correct name should be Iron(III) Sulfide, not Iron(II) Sulfide. All the other pairs are correctly named.
Learn more about Chemistry Compounds here:https://brainly.com/question/37559215
#SPJ11
which feature of the evaporating dish makes it effective in the evaporation process
Answers
Answer:
The precise fit on the ring stand. The ability to hold more substance than a test tube. A shallow bottom to increase the surface area of a liquid, leaving the solid behind.
Explanation:
Q and A!
How does an evaporating dish work?
Evaporating dishes are used to evaporate excess solvents – most commonly water – to produce a concentrated solution or a solid precipitate of the dissolved substance. ... The dish is heated with a Bunsen burner, until only stable precipitate remains, which contains the silica content.
What does a evaporating dish looks like?
The evaporating dish is a small bowl with a spout, usually made of porcelain or borosilicate glass. As its name suggests, it is commonly used to evaporate solvents in a sample. The evaporating dishes we encounter in a chemistry lab usually accommodate small samples.
Good Look On Your Assignment- Joshua Amachee :)You see a gold ring in a flea market that you’d like to have, and the price seems right, so you buy it. On the drive home you wonder if it’s really gold. You do a quick experiment and find it takes 38.7 J of heat to raise the temperature of the 6.00 g ring from 25oC to 75oC. You know that the specific heat of gold is 0.128 J/goC; is your ring really made of gold?
Answers
To determine if the ring is made of gold, we can calculate the expected heat transfer based on the specific heat of gold and compare it to the actual heat transfer observed in the experiment.
The heat transfer equation is given by:
q = m * c * ΔT
Where:
q = heat transfer
m = mass of the object
c = specific heat capacity
ΔT = change in temperature
Given:
m = 6.00 g
c (specific heat of gold) = 0.128 J/g°C
ΔT = 75°C - 25°C = 50°C
Calculating the expected heat transfer using the specific heat of gold:
q_expected = m * c * ΔT
= 6.00 g * 0.128 J/g°C * 50°C
= 38.40 J
The expected heat transfer based on the specific heat of gold is 38.40 J.
Comparing this with the actual heat transfer observed in the experiment, which is 38.7 J, we see that they are very close. This suggests that the ring is likely made of gold, as the measured heat transfer aligns with the expected heat transfer based on the specific heat of gold.
Therefore, based on this experiment, it is likely that the ring is indeed made of gold.
Learn more about heat transfer equation here:
https://brainly.com/question/30526730
#SPJ11
Calculate the molecular mass of glucose
Answers
Answer:
180 u
Hope you could understand.
If you have any query, feel free to ask.
If you are walking down the street and a balanced force is exerted on you what will happen to your motion?
Answers
Answer:
nothing will happen. the motion will not change
Explanation:
Which particles are transferred during a redox reaction
Answers
Answer:
Most oxidation-reduction (redox) processes involve the transfer of oxygen atoms, hydrogen atoms, or electrons, with all three processes sharing two important characteristics: (1) they are coupled—i.e., in any oxidation reaction a reciprocal reduction occurs, and (2) they involve a characteristic net chemical change— .
Electrons will be transferred during redox reaction.
What is electron?The electron is subatomic particles which are placed in surrounding the nucleus. Electrons carry negative charge.
What is redox reaction?Redox reaction involve the transfer of electrons between intermediates. A redox reaction occurs when the oxidation states of the substrate change. The removal of electrons or even a rise in the oxidation state of such a chemical or its atoms is referred to as oxidation. The acquisition of electrons or a lowering in the oxidation number of a chemical or the atoms inside it is referred to as reduction.
Hence the answer will be electron
To know more about redox reaction click here.
https://brainly.com/question/13293425.
#SPJ2
Which causes genetic variations and can result in different alleles? O predation rate O random mutations O competition o environmental changes
Answers
Answer:
B
Explanation:
Answer:
BBBBBBBBB
Explanation:
Applicable in case of strong acid–
(i) gets completely ionized in aqueous solutions
(ii) gives H+ ion in aqueous solution
(iii) sour in taste
Which one is correct?
A
i, ii, iii
B
i, ii
C
ii, iii
D
i, iii
Answers
Answer:
A. i, ii, iii
Explanation:
A strong acid gets completely ionized in aqueous solutions releasing a large number of hydrogen ions. It also has a sour taste.
water containing phenolphthalein will change from colorless to pink with the addition of
Answers
water containing phenolphthalein will change from colorless to pink with the addition of a strong base. An alkaline substance, also known as a base, is a chemical compound that has a pH greater than 7.
Why water containing phenolphthalein change color to pink?
Water containing phenolphthalein will change from colorless to pink with the addition of a basic solution. Phenolphthalein is an indicator commonly used to detect the presence of bases or alkaline substances. In an acidic solution or in pure water (pH below 8.2), phenolphthalein remains colorless. However, when a basic solution is added and the pH rises above 8.2, the phenolphthalein molecule undergoes a chemical change, resulting in a pink color. This color change is often used as a visual cue to indicate the presence of a base in a solution.
tI's important to note that phenolphthalein is not an accurate indicator for all pH ranges. It is only suitable for use in the pH range of 8.2 to 10.0. In solutions with pH values outside this range, phenolphthalein may not produce the expected color change.
Learn more about chemical pH
https://brainly.com/question/172153
#SPJ11
For the α anomer of a D-sugar, the anomeric hydroxyl in a Haworth projection Group of answer choices has an upward projection (on the same side as the terminal CH2OH group). has a downward projection (on the opposite side from the terminal CH2OH group).
Answers
Answer:
The answer is "choice 2".
Explanation:
Its glycosidic fruit juice facility would be a chromosomal hydrogen bond and an aldehyde (or acetone) team generated from the intramolecular creation of the acetals (or ketal).
Its two heterocycles created at the anomeric core from of the 2 potential stereochemical are named anomers, that's why choice "has a downward projection (from terminal CH2OH party on the opposite side)" is correct.
If a 2 kg object produces a 16 N force, what is its acceleration?
Answers
Answer:
f=ma
so a=f/m
a=16N/2kg
a=8m/\(s^{2}\)
K2S is an iconic compound. We need to know the number of particles it breaks into when it dissolves., the Vant Hoff factor. How many particles does K2S break into in water
Answers
The Vant Hoff factor as\(K_2}\)S break into in water is 3
What does vant Hoff factor mean?
The ratio of a substance's mass concentration to the concentration of the particles that are produced when it dissolves is known as the Van't Hoff factor. The Van't Hoff factor describes how much a substance associates or dissociates in a solution.
The ratio of the final moles following dissociation or association to the beginning moles before to dissociation or association of an electrolyte in a solution is known as the Van't Hoff factor. The solute's property governs the number of particles, which is independent of the solution's concentration.
\(K_2}\)S ⇒ 2K+ + \(S_2}\)-
\(K_2}\)S dissolves into 3 particles .
To learn more about Van't Hoff factor use:
brainly.com/question/8837607
#SPJ1
The Vant Hoff factor as \(K_{2}S\) break into in water is 3
What does vant Hoff factor mean?
The ratio of a substance's mass concentration to the concentration of the particles that are produced when it dissolves is known as the Van't Hoff factor. The Van't Hoff factor describes how much a substance associates or dissociates in a solution.
The ratio of the final moles following dissociation or association to the beginning moles before to dissociation or association of an electrolyte in a solution is known as the Van't Hoff factor. The solute's property governs the number of particles, which is independent of the solution's concentration.
\(K_{2}S\) ⇒ 2K+ + -\(S_{2}\)
\(K_{2}S\) dissolves into 3 particles .
To learn more about Van't Hoff factor use:
brainly.com/question/8837607
#SPJ1
Observe: click reset. select the gas collection setup. chemists use this apparatus to collect any gases produced in the reaction. from the reaction flask, gases travel through a long tube and into a cylinder of water. as gases bubble into the cylinder, the water is displaced (removed) until the cylinder is filled with gas. click play and observe the cylinder. was any gas produced in the reaction? [ ]
Answers
Hello. Although it is not possible to see the video to which the question refers, we can see, in the question, that water is removed from the cylinder as a gas is added to the same cylinder. So we can see that a gas was produced in the reaction, through the displacement of water, that is, if the water starts to be removed from the cylinder, it is because the reaction is producing a gas. If the gas is not produced, water will not come out of the cylinder.
Given this equation (linked in screenshot), which of the following is true if 4.53 moles of C6H14 completely reacts with excess oxygen?
A) 0.755 moles CO2 and 0.162 moles H2O will be formed.
B) 27.1 moles CO2 and 31.7 moles H2O will be formed.
C) 12 moles CO2 and 14 moles H2O will be formed.
D) 54.4 moles CO2 and 63.4 moles H2O will be formed.

Answers
The correct answer is option D: 54.4 moles CO₂ and 63.4 moles H₂O will be formed when 4.53 moles of C₆H₁₄ completely reacts with excess oxygen.
What is a chemical reaction?
A chemical reaction is a process that leads to the transformation of one chemical substance to another chemical. It involves breaking and forming of chemical bonds between atoms to create new molecules or compounds.
According to the balanced equation given, 2 moles of C₆H₁₄ react with 19 moles of O₂ to produce 12 moles of CO₂ and 14 moles of H₂O.
Therefore, for 4.53 moles of C₆H₁₄ , the amount of O₂ required for complete reaction would be:
(19/2) x 4.53 = 42.9 moles of O₂
Since excess oxygen is present, all the C₆H₁₄ will react, and the number of moles of CO₂ and H₂O produced will be:
CO₂ = 12 x (4.53/2) = 27.2 moles
H₂O = 14 x (4.53/2) = 31.7 moles
Therefore, the answer is D) 54.4 moles CO₂ and 63.4 moles H₂O will be formed.
To find out more about chemical reactions, visit:
https://brainly.com/question/29762834
#SPJ1
Calculate the molar solubility, in moles per liter, of thallium(I) chromate (TI,CrO4; Kp 8.67x10) in pure water.
Answers
To calculate the molar solubility of thallium(I) chromate (TlCrO4) in pure water, we need to consider the solubility product constant (Ksp) for the compound.
Given:Ksp = 8.67 x 10^(-10)
The solubility product constant expression for TlCrO4 is:
Ksp = [Tl⁺][CrO4²⁻]
Since the compound dissociates into Tl⁺ and CrO4²⁻ ions in water, we can assume that the molar solubility of TlCrO4 is represented by 'x'.
Therefore, at equilibrium, the concentration of Tl⁺ and CrO4²⁻ will both be equal to 'x'.
So, we can write the equilibrium expression as:
Ksp = x * x
Using the given Ksp value, we can set up the equation:
8.67 x 10^(-10) = x * x
Solving for 'x', we take the square root of both sides of the equation:
√(8.67 x 10^(-10)) = x
Calculating this value, we find:
x ≈ 9.32 x 10^(-5) M
Therefore, the molar solubility of thallium(I) chromate (TlCrO4) in pure water is approximately 9.32 x 10^(-5) moles per liter (M).
To know more about molar solubility refer here
brainly.com/question/31493083#
#SPJ11
Directions:Complete the statements in your activity notebook.
I have learned that the solar system ____________________________________
____________________________________________________________________
___________________________________________________________________.
Pls answer it so i can give you (55 points) :]
Answers
The solar system is made up of the sun and everything that orbits around it, including planets, moons, asteroids, comets and meteoroids.
What is the solar system?The sun together with the group of celestial bodies that are held by its attraction and revolve around it
I have learned that the solar system has many planets that can be dangerous for people to live in and there are many things hidden out there that mankind or scientists are not known about, many planets with different kind of names, and there is a lot of stars, comets, asteroids and meteorites everywhere.
Learn more about the solar system here:
https://brainly.ph/question/27909121
#SPJ1
Help. I need to finish this in time for school tomorrow

Answers
5 - Gas
4 - Weight
1 - Elements
3 - Atoms
7 - Mandeleev
2 - Compounds
6 - Carbon Dioxide
8- IUPAC

An unknown compound contains only C , H , and O . Combustion of 7.00 g of this compound produced 16.5 g CO2 and 4.50 g H2O . What is the empirical formula of the unknown compound
Answers
The empirical formula of the unknown compound is C2H5O. This is determined by dividing the number of moles of each element in the products by the lowest number of moles obtained.
To determine the empirical formula of the unknown compound, we need to find the ratio of carbon, hydrogen, and oxygen atoms in the compound.
First, we calculate the number of moles of CO2 and H2O produced in the combustion reaction. The molar mass of CO2 is 44 g/mol, and the molar mass of H2O is 18 g/mol.
Number of moles of CO2 = 16.5 g / 44 g/mol ≈ 0.375 mol
Number of moles of H2O = 4.50 g / 18 g/mol = 0.25 mol
Next, we determine the number of moles of carbon, hydrogen, and oxygen in the unknown compound. In the combustion reaction, each CO2 molecule contains one carbon atom and each H2O molecule contains two hydrogen atoms.
Number of moles of carbon = 0.375 mol (from CO2)
Number of moles of hydrogen = 0.25 mol × 2 = 0.5 mol (from H2O)
Number of moles of oxygen = 0.375 mol × 2 = 0.75 mol (from CO2)
Now we need to find the simplest whole-number ratio of carbon, hydrogen, and oxygen atoms. To do this, we divide each number of moles by the smallest number of moles, which is 0.25 mol.
Number of moles of carbon in the simplest ratio = 0.375 mol / 0.25 mol = 1.5 ≈ 2
Number of moles of hydrogen in the simplest ratio = 0.5 mol / 0.25 mol = 2
Number of moles of oxygen in the simplest ratio = 0.75 mol / 0.25 mol = 3
Therefore, the empirical formula of the unknown compound is C2H5O, which represents the simplest whole-number ratio of carbon, hydrogen, and oxygen atoms in the compound.
To learn more about compound click here:
brainly.com/question/14117795
#SPJ11
Mobile water supply operations, such as water shuttles or relay pumping, must be performed:_____.
Answers
Mobile water supply operations, such as water shuttles or relay pumping, must be performed In rural areas without public water distribution systems
A distribution systems includes all of the centers and devices connecting a transmission device to the customer's device. A traditional distribution device can consist of: Substations. Distribution Feeder Circuits. Switches.
Distribution systems, additionally referred to as dispensed computing, is a device with more than one additive placed on extraordinary machines that speak and coordinate movements so that it will seem like an unmarried coherent device to the end-user.
A dispensed device is computing surrounding wherein diverse additives unfold throughout more than one computer (or different computing gadgets) on a network. These gadgets cut up the work, coordinating their efforts to finish the task extra effectively than if an unmarried tool were accountable for the task.
Learn more about distribution systems here;
https://brainly.com/question/27905732
#SPJ4
Help me please What makes water filters better
Answers
Answer:
well according to salesman they take out all and out all bacteria that is in the water that the water filters installed in the systems already don't take out so in other wordsthere and the water filter takes that out
Determine where each type of cleaning solution should be discarded after use.Water used to rinse detergent out of a flask _____ sink or waste containerSolvet used to rinse chemicals out of a beaker_____ sink or waste containerAcid solution used to clean a crucible___________ sink or waste container
Answers
Water used for washing purposes can be discarded into the sink as it does not affect the water pollution.
But in the case of organic solvent and acid solution they can be discarded into waste containers for proper disposal.
As we know that organic solvent and acid solution are hazardous for aquatic life. Their discopsal is a major concern.
What is water?
Water, a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid and solid states. It is one of the most abundant and basic compounds. At room temperature, the tasteless and odorless liquid has an important ability to dissolve many other substances.To know more about water, click the link given below:
https://brainly.com/question/17435626
#SPJ4
Need help fast please!
Find the mass of 4.56 mols of LaPO4
Answers
Answer:
Mass = 1080.26 g
Explanation:
Given data:
Number of moles of LaPO₄ = 4.56mol
Mass in gram = ?
Solution:
Formula:
Number of moles = mass/molar mass
Molar mass of LaPO₄ = 236.9 g/mol
by putting values,
4.56 mol = mass/ 236.9 g/mol
Mass = 4.56 mol × 236.9 g/mol
Mass = 1080.26 g
Question 15 Why do atoms transfer or share electrons? (Select all that apply)
A.To decrease density.
B.To form more interesting structures.
C.To obtain an octet.
Answers
Atoms transfer or share electrons to obtain octet (option C).
What is octet?Octet rule in chemistry refers to a rule stating that atoms lose, gain, or share electrons in order to have a full valence shell of 8 electrons.
Hydrogen is excluded from this rule because it can hold a maximum of 2 electrons in its valence shell.
During chemical reactions, atoms of elements combine with other atoms and lose, gain or share their electrons so as to obtain this octet state of electrons.
Learn more about octet rule at: https://brainly.com/question/865531
#SPJ1
Q-3 Determine the fugacity in atm for pure ethane at 310 K and 20.4 atm and change in the chemical potential between this state and a second state od ethane where temperature is constant but pressure is 24 atm.
Answers
The fugacity in atm for pure ethane at 310 K and 20.4 atm is given by the equation: f = 20.4 exp (-Δg1/RT). The change in chemical potential between this state and a second state of ethane where the temperature is constant but the pressure is 24 atm is -0.0911RT.
Fugacity is a measure of the escaping tendency of a component in a mixture, which is defined as the pressure that the component would have if it obeyed ideal gas laws. It is used as a correction factor in the calculation of equilibrium constants and thermodynamic properties such as chemical potential. Here we need to determine the fugacity in atm for pure ethane at 310 K and 20.4 atm and the change in the chemical potential between this state and a second state of ethane where the temperature is constant but the pressure is 24 atm. So, using the formula of fugacity: f = P.exp(Δu/RT) Where P is the pressure of the system, R is the gas constant, T is the temperature of the system, Δu is the change in chemical potential of the system. Δu = RT ln (f / P)The chemical potential at the initial state can be calculated using the ideal gas equation as: PV = nRT
=> P
= nRT/V
=> 20.4 atm
= nRT/V
=> n/V
= 20.4/RT The chemical potential of the system at the initial state is:
Δu1 = RT ln (f/P)
= RT ln (f/20.4) Also, we know that for a pure substance,
Δu = Δg. So,
Δg1 = Δu1 The change in pressure is 24 atm – 20.4 atm
= 3.6 atm At the second state, the pressure is 24 atm.
Using the ideal gas equation, n/V = 24/RT The chemical potential of the system at the second state is: Δu2 = RT ln (f/24) = RT ln (f/24) The change in chemical potential is Δu2 – Δu1 The change in chemical potential is
Δu2 – Δu1 = RT ln (f/24) – RT ln (f/20.4)
= RT ln [(f/24)/(f/20.4)]
= RT ln (20.4/24)
= - 0.0911 RT Therefore, the fugacity in atm for pure ethane at 310 K and 20.4 atm is:
f = P.exp(Δu/RT)
=> f
= 20.4 exp (-Δu1/RT)
=> f
= 20.4 exp (-Δg1/RT) And, the change in the chemical potential between this state and a second state of ethane where the temperature is constant but pressure is 24 atm is -0.0911RT. Therefore, the fugacity in atm for pure ethane at 310 K and 20.4 atm is given by the equation: f = 20.4 exp (-Δg1/RT). The change in chemical potential between this state and a second state of ethane where the temperature is constant but the pressure is 24 atm is -0.0911RT.
To know more about chemical potential visit:-
https://brainly.com/question/31100203
#SPJ11
Which of the following is not a function of the cell?
Sleeping
Protection
All of the above
Reproduction