Answers
Related Questions
True or false. An ion is an electrically neutral atom that has the same number of protons and neutrons
Answers
Answer:
False
Explanation:
Atoms are neutral Ions carry a charge. Take a look at the groups in the periodic table for extra help in understanding this.
please help me please it's urgent

Answers
Answer:
Paraphrase this! I got this online!1.A a Chemical Reaction is a process that involves rearrangement of the molecular or ionic structure of a substance, as opposed to a change in physical form or a nuclear reaction.
1.A Chemical reactions involve breaking chemical bonds between reactant molecules (particles) and forming new bonds between atoms in product particles (molecules)
1A.Chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other stable species that make up the familiar substances of the everyday world.
2a.A gas evolution reaction is a chemical reaction in which one of the end products is a gas such as oxygen or carbon dioxide. Gas evolution reactions may be carried out in a fume chamber when the gases produced are poisonous when inhaled or explosive.
2b.a color change is usually an indicator that a reaction is occurring
2c.Processes involved in changes of state include melting, freezing, sublimation, deposition, condensation, and evaporation
3A.When you increase the pressure, the molecules have less space in which they can move. That greater density of molecules increases the number of collisions
3B.As an object gets hot, its atoms and molecules vibrate. As they vibrate, they release energy in the form of light. ... In chemiluminescence, energy from a chemical reaction excites the electrons of a substance
3C. Catalysis is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst. Catalysts are not consumed in the reaction and remain unchanged after it.
3D.Most chemical reactions involve the breaking and formation of chemical bonds. It takes energy to break a chemical bond but energy is released when chemical bonds are formed. If more energy is released than consumed, then the chemical reaction evolves heat and is said to be exothermic. ... Most reactions are exothermic.
Paraphrase this! I got this online!Paraphrase this! I got this online!Paraphrase this! I got this online!Why is there something blocking the answer?
Answers
Answer: the answer you are looking for does not exist
Explanation:
What is the lengthening of a muscle without damage? And what is the shortening of a muscle?
Answers
Answer:
Stretching is the lengthening and contraction is the shortening
Explanation:
Unless it's asking for eccentric as the stretching and concentric as the shortening
If 11.5 ml of vinegar sample (d=1g/ml) is titrated with 18.5 ml of standardized Sodium hydroxide
solution. What is the percentage of acetic acid (by mass) in the vinegar
Answers
Answer:
4.83% of acetic acid in the vinegar
Explanation:
The neutralization reaction of acetic acid (CH₃COOH) with sodium hydroxide (NaOH) is:
CH₃COOH + NaOH → CH₃COO⁻Na⁺ + H₂O
Assuming the sodium hydroxide solution has a concentration of 0.500M, moles used in the neutralization reaction are:
0.0185L × (0.500mol / L) = 0.00925 moles of NaOH = 0.00925 moles CH₃COOH
Because 1 mole of acid reacts per mole of NaOH
Using molar mass of acetic acid (60g/mol):
0.00925moles CH₃COOH ₓ (60g / mol) = 0.555g of CH₃COOH
As mass of vinegar sample is 11.5g (d = 1g/mL), percentage of acetic acid by mass is:
0.555g CH₃COOH / 11.5g vinegar × 100 = 4.83% of acetic acid in the vinegar
What are non-examples of a nucleus
Answers
Answer:n a red blood cell, the control center is the nucleus. A mitochondria is not a nucleus. It is another organelle that produces energy for the cell.
Explanation:
2,4-Dimethylpent-2-ene undergoes an electrophilic addition reaction in the presence of HBr to form 2-bromo-2,4-dimethylpentane. Complete the mechanism of this addition and draw the intermediates formed as the reaction proceeds.
Answers
Answer:
See figure 1
Explanation:
In this case, we have to start with the ionization reaction of HBr to produce the hydronium ion (\(H^+\)) and the bromide ion (\(Br^-\)). Then the double bond in the alkene can attack the hydronium ion to produce a carbocation. The most stable carbocation would be the tertiary one, therefore we have to put the positive charge in the tertiary carbon. Then, the bromide attacks the carbocation to produce the final halide.
See figure 1
I hope it helps!

the # molecules in 52.1 grams of NH4OH
Answers
Answer:
Explanation:
Hey there!
From question;
The molecular mass of NH₄OH is 35.
Avogadro number = 6.023*10²³ molecules.
Then,
From mole concept,
Molecular mass = Avogadro number
So, 35 grams contains 6.023*10²³ molecules.
1 gram contains \(\frac{ 6.023*10^{23} }{35}\) molecules.
52.1 grams contains \(\frac{6.023*10^{23} }{35} *52.1\) molecules.
Therefore, 52.1 grams contains 8.96*10²³ molecules.
Hope it helps!
Ca(OH)2 + 2HNO3 → Ca(NO3)2 + 2H2O
Answers
Answer: You have it right
Explanation:
you put the two on the H2O to make it 2H2O and A two on the HNO3 to make it 2HN03 To make it balanced, good job
which method is adopted in the seperation of lead chloride from water
Answers
Explanation:
by dissolving the mixture of lead sulphate and lead chloride in water we can separate the two. after dissolving the mixture on water lead sulphate can be obtained as the solid that 's left behind lead chloride can be recovered by evaporating.
Nonane and 2,3,4-trifluoropentane have almost identical molar masses, but nonane has a significantly higher boiling point. Which of the following statements best helps explain this observation?
Answers
Compared to 2,3,4-trifluoropentane, the nonane's carbon chains are longer.
In chemistry, what exactly is a molar mass?A substance's molar mass is defined as its molecular weight in grams. By adding the molar masses of a substance's constituent atoms, we may get the substance's molar mass. Then, to convert between mass and the quantity of moles of the material, we may utilize the computed molar mass.
A molar mass is determined in what way?Adding the atomic masses of a particular substance results in the calculation of molar mass. Below each element's symbol on the periodic table is a designation of the mass of that specific element. The molar mass is obtained by averaging the atomic masses obtained from the periodic table.
To know more about Molar mass visit:
https://brainly.com/question/22997914
#SPJ1
Which statement is true about a neutral solution? (5 points)
Its pH is less than 7.
Its pH is greater than 7.
It has the same concentration of hydronium and hydroxide ions.
It has a greater concentration of hydroxide ion than hydronium ions.
Answers
The statement "It has the same concentration of hydronium and hydroxide ions" is true about a neutral solution.
What is a neutral solution?If you've ever wondered what constitutes a neutral solution - it's one with an ideal pH balance set at precisely 7. Referred to as a 'neutral' because it doesn't lean towards acidity or alkalinity due to an equal concentration rate between hydronium ions (H+) and hydroxide ions (OH-).
Noteworthy examples can include distilled water, pure water, blood seawater, milk of magnesia among others.
Learn about neutral solution here https://brainly.com/question/21444245
#SPJ1
5. An unknown metal has a mass of 4.67 g. It is heated to 95.1°C and then placed in a
calorimeter that contains 24.3 g of water at 21.7°C. The metal and water both reach
a final temperature of 24.6°C. What is the specific heat of this metal? What is the
unknown metal?

Answers
The unknown metal with C(metal) of 0.90J/gC and mass of 4.67g is aluminum.
CalorimeterThe metal's specific heat is calculated using the heat equation. It is important to note that the total heat (Q), which is the sum of the two heats (Qwater and Qmetal), is equal to zero at equilibrium.
Now, Q(total)=Q(water)+Q(metal)
0=m(water)
The specific heat of water, C(water), is equal to 4.18 J/g, while the other two components are water and metal.
The temperature of a metal is known as C(metal).
With the given values all substituted, we obtain 0=m(water) C(water)T(water) +m(metal).
CmetalΔTmetal=(24.3g)(4.184J/g°C) (24.6°C−21.7°C)+(4.67g) (Cmetal)(24.6°C−95.1°C)
The metal's specific heat is given by the equation C(metal)=0.90J/gC, which is simplified by placing C(metal) on one side of the equation.
Part (b):As a result, aluminum is the metal.
For more information on calorimeter kindly visit to
https://brainly.com/question/4802333
#SPJ1
What property can be easily measured in solids, liquids, and gases? (2 points)
Group of answer choices
The temperature of solids, liquids, and gases can be easily measured.
The texture of solids, liquids, and gases can be easily measured.
The color of solids, liquids, and gases can be easily observed.
The texture and temperature can be easily measured for solids, liquids, and gases.
Answers
Answer:
I think the answer is A
Explanation:
the temperature of solids , liquids and gases can be easily measured
What is the meaning of friction
Answers
Explanation: the resistance that one surface or object encounters when moving over another.
or
the action of one surface or object rubbing against another.
Answer: a force that resists the motion of one object against another
Phosphine (PH3) can be prepared by the reaction of calcium phosphide , Ca3P2: based on this equation : Ca3P2 + 6H2O —-> 3 Ca(OH)2 + 2 PH3
Based on this equation , which of the following statements are correct?
(a) one mole of Ca3P2 produces 2 mol of PH3
(b) one gram of Ca3P2 produces 2g of PH3
(c) Three moles of Ca(OH)2 are produced for each 2 mol of PH3 produced
(d) the mole ratio between phosphine and calcium phosphide is 2 mol PH3 over 1 mol Ca3P2
Answers
Taking into account the reaction stoichiometry, you can observe that:
one mole of Ca₃P₂ produces 2 mol of PH₃.the mole ratio between phosphine and calcium phosphide is 2 mol PH₃ over 1 mol Ca₃P₂.Reaction stoichiometryIn first place, the balanced reaction is:
Ca₃P₂ + 6 H₂O → 3 Ca(OH)₂ + 2 PH₃
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Ca₃P₂:1 mole H₂O: 6 molesCa(OH)₂: 3 molesPH₃: 2 molesThe molar mass of the compounds is:
Ca₃P₂: 182 g/mole H₂O: 18 g/moleCa(OH)₂: 74 g/molePH₃: 34 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Ca₃P₂: 1 mole ×182 g/mole= 182 grams H₂O: 6 moles× 18 g/mole= 108 gramsCa(OH)₂: 3 moles ×74 g/mole= 222 gramsPH₃: 2 moles ×34 g/mole= 68 gramsCorrect statementsThen, by reaction stoichiometry, you can observe that:
one mole of Ca₃P₂ produces 2 mol of PH₃.the mole ratio between phosphine and calcium phosphide is 2 mol PH₃ over 1 mol Ca₃P₂.Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
According to the Brinsted -Lowry. what is an acid ? what is a base ?
Answers
Answer:
According to the Brønsted definition, an acid is a substance capable of donating a proton, and a base is a substance capable of accepting a proton. ... The species giving up the proton is HCl, an acid. The species accepting the proton is water, the base. The species Cl- is the conjugate base of HCl.
The formula for water is H₂O meaning there are 2 Hydrogen atoms and 1 oxygen. What is the atomic mass of one molecule to the nearest hundredth?
A) 17.99
B) 16.99
C) 15.99
D) 18.99
Answers
\( { \bf\implies 17.99u}\)
Step-by-step explanation:We know that water is the combination of two hydrogen atoms and one oxygen atoms.To find atomic mass of water (\(\bf{H_2O}\))
Atomic mass of Hydrogen × 2 + Atomic mass of oxygen × 1We know that,
Atomic mass of Hydrogen = 1Atomic mass of Oxygen = 15.99\(\mapsto\)1 × 2 + 15.99 × 1
\(\mapsto\)2 + 15.99
\(\mapsto\) 17.99u Ans.
Additional information :Learn the Period table of Elements to solve this type of questions. It is very important. I attached the period table of elements. Learn it and you are able to solve it.
what protect the contents of the nucleus
Answers
Answer:
The nucleus contains all of the genetic material for a eukaryotic cell, but this genetic material needs to be protected.
Explanation:
Hopes this helps
❤(っ^▿^)
100 grams of reactants completely react to form the products in a reaction how many grams of product are produced
Answers
If 100 grams of reactants completely react to form the products in a reaction, the amount of products produced will also be 100 grams.
Law of conservation of massThe law of mass conservation states that in a closed system, the total mass remains constant before and after a chemical reaction. This means that the mass of the reactants is equal to the mass of the products.
To solve the given problem, the law of mass conservation can be applied by assuming that the reaction is complete.
Thus, if 100 grams of reactants completely react, it implies that the total mass of the products formed will also be 100 grams, assuming no other substances are involved in the reaction.
More on mass conservation can be found here: https://brainly.com/question/14549722
#SPJ1
How many moles of gold are there in 28 grams of gold?
Answers
Answer: There are approximately 0.142 moles in 28 grams of Gold.
Explanation:
To calculate the number of moles 28 grams of Gold, we need to know the molar mass of gold. The molar mass of gold (Au) is approximately 197 grams per mole.
We can use the formula:
Number of moles = Mass (in grams) / Molar mass
Substituting the given values:
Number of moles = 28 g / 197 g per mole
Number of moles ≈ 0.142 moles (approx.)
Therefore, there are approximately 0.142 moles of gold in 28 grams.
For more questions on Molar mass and moles, see :
https://brainly.in/question/11731
Two identical automobiles are racing towards each other. One vehicle is going 30 mph, the other is going 60 mph. What will happen when the two vehicles collide, and why? What would happen if the two cars were moving at identical speeds?
Answers
When the two vehicles collide, then vehicle with slow speed will pushes back.
How we define speed?Speed of any moving body is define as the distance travelled by that body in per unit of time.
As it is mentioned that one car has a speed of 30 mph and another one has a speed of 60mph, if both of these cars get collide then the car with high speed pushed back the car with low speed.
Hence the car with slow speed pushed back.
To know more about speed, visit the below link:
https://brainly.com/question/6504879
#SPJ1
What is the main molecule that provides the energy to produce ATP?
Answers
Answer: glucose
Explanation:
When two atoms are being held together by an ionic bond, which of the following statements is accurate?
a. Each of the two atoms had a full outer orbital before the bond occurred.
b. The two atoms share an electron.
c. One atom is now positively charged and the other is negatively charged.
d. The two atoms have identical atomic numbers.
Answers
Correct option is c) One atom is now positively charged, and the other is negatively charged. This is due to nature of ionic bond.
An ionic bond is formed when one or more electrons are transferred from one atom to another, resulting in the formation of two oppositely charged ions. In an ionic bond, the electron(s) transferred from one atom are accepted by the other atom, leading to the formation of cations and anions.
The atom that loses an electron becomes a positively charged ion (cation), and the atom that gains an electron becomes a negatively charged ion (anion). The opposite charges of the ions attract each other and form an ionic bond between the two atoms.
Ionic bonds usually occur between atoms of different elements with a significant difference in electronegativity. Ionic compounds generally have high melting and boiling points, are solid at room temperature, and are usually soluble in water.
In contrast to an ionic bond, a covalent bond is formed when atoms share electrons, as in the case of molecular compounds. In a covalent bond, atoms do not become charged. Instead, they share electrons in a way that allows them to complete their valence shells.
Learn more about ionic bond here:
https://brainly.com/question/11527546
#SPJ4
Barium nitrate (Ba(NO3)2) reacts with sodium chloride (NaCl) in a double replacement (displacement) reaction, shown below.
Ba(NO3)2(aq)+NaCl(aq)-->???
How many grams of barium salt are produced when a solution containing 21.7 g of Barium nitrate is mixed with a solution containing excess sodium chloride?
Use 261.34 as the molar mass for barium nitrate. Round to three significant digits.
Answers
Answer:
you know that they will be a displacement reaction that will form a barium salt:
Ba(NO3)2+ 2NaCl--> BaCl2 + 2NaNO3
So now that we have that formula and the molecular weight we can determine how much salt will be made. So here we convert the grams to moles
(42.3g Ba(NO3)2)*(1 mole/261.34g) = 0.16185 mol
In the molecular formula we know that 1 mole of Barium nitrate will create 1 mole of Barium chloride, so in this case (in a perfect world) you should get 0.16185 mole of barium chloride (208.23 g/mol) that we then have to convert to grams.
(0.16185 mol BaCl2) * ( 208.23 g/mol) = 33.7037 g of Barium Chloride (rounded to 3 significant digits = 33.7g)
Identify the limiting reactant in the reaction of methane (CH4) and carbon tetrachloride to form CH2Cl2, if 2.96 g of CH4 and 32.0 g of CCl4 are combined. Determine the amount (in grams) of excess reactant that remains after the reaction is complete.
Answers
Answer:
d
Explanation:
yes
Atom A and B both have 10 protons A have 10 B has 11 neutrons, which statement is true A,b have the same element, theyvhave differnet elements, if they have the same mass, only atom b is a isotope
Answers
Atom A and B both have 10 protons A have 10 B has 11 neutrons is A and B are isotopes of same element.
Isotopes are the atoms of same elements , that has same number of proton but different number of neutrons , mass no. is depends on neutrons present in an atom. the given situation is :
number of proton number of neutron
Atom A 10 10
Atom B 10 11
Isotopes are the members of the same family of same elements.
Thus, Atom A and B both have 10 protons A have 10 B has 11 neutrons is A and B are isotopes of same element.
To learn more about Isotopes here
https://brainly.com/question/17335691
#SPJ1
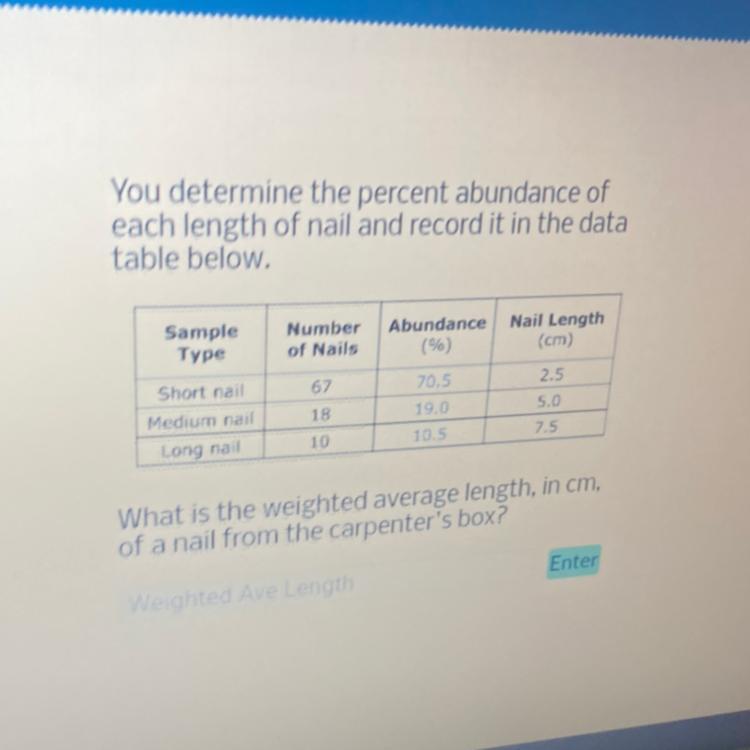
What is percent abundance of 18 medium nails 5 cm long?
Answers
From the attached image, the percentage abundance of 18 medium nails 5 cm long is 19%
Understanding Percentage AbundanceThe percent abundance refers to the proportion or percentage of a certain type or category within a given sample or population.
In the case of 18 medium nails that are 5 cm long, we have the information presented in the table and we do not need to do any mathematical calculations.
Learn more about percentage abundance here:
https://brainly.com/question/6844925
#SPJ1
PLEASE HELP ME!!!!!!!!!!!!!!!!!!!
WRITE A SHORT PASSAGE ON HOW FIBRES ARE SPUN
HELPPP WILL MARK AS BRAINLIEST
Answers
Answer: Spun yarn is made by twisting staple fibers together
To make a cohesive thread Or “single”. Twisting fibers, into yarn in the process called spinning can be dated back to the upper Paleolithic. Yarn spinning was one of the first processes to be industrialized.
Explanation:
when an electron jumps from a larger orbit to a smaller orbit within an atom, it changes from a energy level to a energy level and a photon is .
Answers
The electron changes from a higher energy level to a lower energy level, and a photon is emitted.
What is electrons?
Electrons are the smallest and most fundamental particles that make up matter. They are negatively charged particles that exist in the orbits of atoms and molecules and that participate in chemical reactions. Electrons are found in all atoms, and determine the chemical properties of the atom. They are also responsible for electricity and magnetism, and can be used to create electrical current in circuits. Electrons have a very small mass and move around the nucleus of an atom very quickly. In addition, electrons can be excited by certain energies, causing them to move to higher energy levels, where they can then be used to create electrical current.
Therefore, The electron changes from a higher energy level to a lower energy level, and a photon is emitted.
To learn more about electrons
Here: https://brainly.com/question/26084288
#SPJ4