The research and design team of a car manufacturing company is developing a new safety product that automatically
tests a car's brakes. When the team discusses how exactly they plan to determine whether the new technology is
successful or not, what stage of technological design are they in?
O first
O second
third
O fourth
Answers
The team should discuss the plan to determine whether the new technology is successful or not in the fourth stage. The correct option is D.
What are different stages of technology testing?When a company manufactures any item, he test it before sending it to the market. This is called technology testing. The test goes from four stages. Here, the item is car's break.
Thus, the correct option is D, fourth stage.
Learn more about technology testing
https://brainly.com/question/16819816
#SPJ5
Related Questions
A newspaper started an online version of its paper 14 years ago. In a recent presentation to stockholders, the lead marketing executive stated, “The revenues for online ads are more than double that of the revenues for printed ads.”
Answers
Answer:
According to the graph, during year 14, revenue from online ads is approximately $4.2 million, while revenue from online ads has more than doubled revenue from printed ads (at least by year 14)
Both curves intersect between the seventh and eighth year, I would say around the middle of year 7, and both online ads and printed ads generated an approximate revenue of $2.2 million each
Explanation:
□ 4. In a closed container, the temperature is increased by 50%. Which of the following will happen to the gas inside the container?
O It will remain unchanged.
The molecules will move more slowly.
The molecules will exert less pressure on the container walls
O The molecules will move more quickly.
Answers
Answer: The molecules will move more quickly.
Explanation:
As temperature increases, the kinetic energy of the particles increases, causing them to move more quickly.
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
A 10.0 g piece of hot metal at 300. °C was dropped into a 150.0 g sample of cooler room temperature water that was initially 25.0 °C. If 6.25 kJ of heat was transferred, what was the final temperature of the water?What was the specific heat of the metal?
Answers
Answer:
The final temperature of water is 34.92 ⁰C
The specific heat of the metal is 2357.78 J/kg⁰C
Explanation:
Given;
mass of the hot metal, m = 10.0 g = 0.01 kg
temperature of the hot metal, \(t_m\) = 300 °C
mass of water, \(m_w\) = 150 g = 0.15 kg
initial temperature of the water, \(t_w_i\) = 25.0 °C
heat lost by the hot metal = heat gained by water
Q = 6.25 kJ = 6250 J
let the final temperature of water = T
\(Q = m_w C_p_w (T-t_w_i)\\\\T-t_w_i = \frac{Q}{m_wC_p_w}\\\\ T= \frac{Q}{m_wC_p_w} + t_w_i\\\\T = \frac{6250}{(0.15)(4200)} + 25\\\\T = 34.92 ^0 C\)
The final temperature of water is also the equilibrium temperature.
The specific heat of the metal is given by
\(Q = mC_p_m (300 - T)\\\\C_p_m = \frac{Q}{m(300 - T)}\\\\ C_p_m = \frac{6250}{0.01(300 - 34.92)}\\\\ C_p_m = 2357.78 \ J/kg ^0C\)
how much heat is needed to melt 100.0 grams of ice that is already at 0 c group of answer choices 33400j 226000 j 33400j 226000 j
Answers
100g of ice will require 33,400 J to melt because the specific heat required to melt ice is 334 J/g. Since water has a specific heat of vaporization of 2230 J/g, evaporating 100g of water will require 223,000 J.
What does science mean when it refers to heat?Heat is the result of the movement of kinetic energy from one media or item to another, or from the energy source to either a medium or object. Radiation, conduction, & convection are the three possible mechanisms for this type of energy transmission.
How can you locate heat in chemistry?The equation q = mcT, where m is the sample mass, c is the specific heat, and T is the temperature change, can be used to determine how much heat is absorbed or lost by a sample (q).
To know more about Heat visit:
https://brainly.com/question/1429452
#SPJ4
Report accidents to your teacher,no matter how they may seem
Answers
Answer:
I mean If you're asking a question, then yes.
convert 7.1 grams of potassium to atoms.
Answers
Answer:
In 7.1g of potassium, there are 1.09 * 10^23 atoms
Explanation:
HERE, we want to get the number of atoms on 7.1g of potassium
The first thing we have to do here is to convert the given mass to moles
We can do this by dividing the mass by the atomic mass unit of potassium
The atomic mass unit of potassium is 39.1 amu
We can get the number of moles mathematically as follows:
\(\frac{7.1}{39.1}\text{ = 0.1816 moles}\)Finally,we can get the number of atoms
In 1 mole of a substance, the number of atoms is:
\(6.02\text{ }\times10^{23\text{ }}\text{ atoms}\)To get the number of atoms in 0.1816 mole, we simply multiply the two as follows:
\(0.1816\times\text{ 6.02}\times10^{23}\text{ = 1.09 }\times10^{23}\text{ atoms}\)
Ammonia is a covalent bonded compound because... I need helpppp!!!!
Answers
Ammonia is in in fact a polar covenant bond because nitrogen is attached to hydrogen but the electrons between nitrogen and hydrogen are not shared equally because nitrogen has a different electronegativity value than hydrogen.
why is time an independent variable
Answers
HELP PLSSSS
In terms of their chemical properties (such as how they react with things), how do the elements in a group compare to the other elements in that group? For example, Sodium (Na) and Potassium (K) are both in the same group, what does this mean about their chemical properties?
Answers
Answer:
they're called families
Explanation:
the way they are grouped depends on how many valence electrons they have and similar chemical properties. for example: alkaline metals, alkaline earth metals, halogens, etc. :)
with an atomic number of 11, which of these elements gets its symbol from the latin word natrium?
Answers
The element with an atomic number of 11 that gets its symbol from the Latin word "natrium" is Sodium. Its symbol is "Na".
The symbol for sodium is Na, which is derived from the Latin word natrium. Sodium is a soft, silvery-white, highly reactive metal that is a member of the alkali metal group. It is an important element for many biological processes and is commonly found in salt (sodium chloride).
The other elements listed in the question are chlorine, iron, and nitrogen. Chlorine has an atomic number of 17, iron has an atomic number of 26, and nitrogen has an atomic number of 7. None of these elements gets their symbol from the Latin word natrium.
For more question on atomic number click on
https://brainly.com/question/11353462
#SPJ11
Probable question would be
with an atomic number of 11, which of these elements gets its symbol from the latin word natrium?
Sodium
Chlorine
Iron
Nitrogen
HELP !!
Convert 84,520 g of Ne to atoms.
Answers
Answer:
2.53 x 10^24
Explanation:
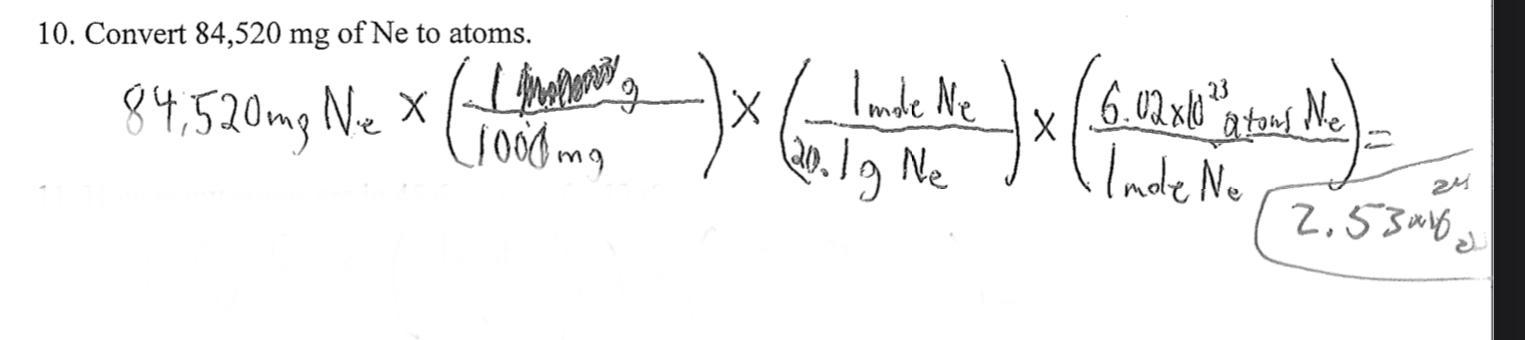
Describe and explain the trend in atomic a.radius within the group.Explain the difference between the size of b.the atoms and the size of the ions.
Answers
Answer:
A. The atomic radius increases (in a group) with the increasing atomic number. This is because atomic size generally increases from top to bottom within a group because the greater the number of protons means the greater amount of electrons. The amount of electrons in orbitals determine an atom's size/radius.
B. Since Group 1A (Alkali Metals) are metals, they tend to form cations. Cations are always smaller than their original atom because the greater positive charge from the nucleus closes in the space between it and the electrons, thus "shrinking" its size. This is why the ionic radii are smaller than the atomic radii of the same element.
I hoped this helped <3
Explanation:
When comparing bromine to chlorine, bromine has
A. Fewer protons in its nucleus.
B. A weaker effective nuclear charge
C. More valence electrons
D. More electron shielding
Answers
Explanation:
I'm pretty sure the answer is D
PREPARATION OF BASES
Answers
The preparation of bases involves several methods that are used to create substances with basic or alkaline properties are Reaction of metal with water, Reaction of metal oxide with water, Neutralization reaction, Ammonia gas dissolving in water and Partial neutralization of a strong base with a weak acid.
Reaction of metal with water: Certain metals, such as sodium or potassium, react with water to form hydroxides. For example, sodium reacts with water to produce sodium hydroxide (NaOH).
Reaction of metal oxide with water: Metal oxides, such as calcium oxide (CaO) or magnesium oxide (MgO), can be added to water to form metal hydroxides. This process is known as hydration. For instance, when calcium oxide reacts with water, it forms calcium hydroxide (Ca(OH)2).
Neutralization reaction: Bases can be prepared by neutralizing an acid with an appropriate alkaline substance. This involves combining an acid with a base to form water and a salt. For example, mixing hydrochloric acid (HCl) with sodium hydroxide (NaOH) results in the formation of water and sodium chloride (NaCl).
Ammonia gas dissolving in water: Ammonia gas (NH3) can dissolve in water to form ammonium hydroxide (NH4OH), which is a weak base.
Partial neutralization of a strong base with a weak acid: Mixing a strong base, such as sodium hydroxide (NaOH), with a weak acid, like acetic acid (CH3COOH), results in the formation of a base with a lesser degree of alkalinity.
These methods are utilized in laboratories, industries, and various applications where bases are required, such as in the production of cleaning agents, pharmaceuticals, and chemical reactions. Each method has its own advantages and specific applications depending on the desired base and its properties.
The question was incomplete. find the full content below:
What are the various methods involved in the preparation of bases?
Know more about Neutralization Reaction here:
https://brainly.com/question/23008798
#SPJ8
which of the following is the least likely zone of formation for a large air mass?
Answers
The least likely zone of formation for a large air mass is the equator. Large air masses form due to differences in temperature and pressure between different regions.
The equator is an area where temperatures are relatively consistent throughout the year and there are not significant differences in pressure systems, making it less likely for a large air mass to form. On the other hand, areas near the poles or where there are large landmasses or bodies of water can have significant differences in temperature and pressure, making them more likely to form large air masses.
Air masses typically form in regions with consistent temperature and humidity conditions, such as polar, tropical, and continental areas. The equatorial region is less likely to form large air masses because it experiences strong solar heating, high humidity, and a lot of weather variability, which prevents the development of stable, uniform air masses.
To know more about mass visit:
https://brainly.com/question/11954533
#SPJ11
Identify the phrases that generally apply to molecular compounds. A. Contains metals and nonmetals B. Are often fades or liquids C. Have low melting points D. Contain ionic bonds E. Use covalent bonding
Answers
Answer is option C) Have low melting points.
Explanation:
As is common knowledge, small molecules make up molecular compounds, and these small molecules are held together by an intermolecular force. The intermolecular tensions in this situation are incredibly weak and simple to overcome when breaking it. Therefore, molecular compounds have low melting and boiling points because of weak intermolecular interactions.
What are molecular compounds?Chemical substances known as molecular compounds assume the shape of distinct molecules. Examples include common substances like carbon dioxide and water. These substances differ significantly from ionic substances like sodium chloride. When metal atoms give up one or more of their electrons to nonmetal atoms, ionic compounds are created. The resultant cations and anions are drawn to one another electrostatically.
To learn more about molecular compounds visit;
https://brainly.com/question/23088724
#SPJ4
A balloon is filled to a volume of 2.00 L with 3.50 moles of gas at 25
°C. With pressure and temperature held constant, what will be the
volume of the balloon if 1.60 moles of gas are added?
Answers
ANSWER;
2.914 L
Explanation:
A balloon is filled to a volume of 2.00 L with 3.50 moles of gas at 25
°C. With pressure and temperature held constant, what will be the
volume of the balloon if 1.60 moles of gas are added?
WE HAVE INCREASED THE NUMBER OF PARTICLES THE BALLOON BY
45.7%
ALL OTHER THINGS EQUAL, WE HAVE INCREASED THE VOLUME BY
45.7%
THE NEW VOLUME SHOULD BE 2.00 X 1.457= 2.914 L
What is the TLV for sodium hydroxide? Will mark brainliest!
Answers
Answer:
you could've googled it but here.
Explanation:
The former OSHA limit for sodium hydroxide (also known as caustic soda or Iye) was 2 mg/m3 as an 8-hour TWA. OSHA proposed a 2-mg/m3 ceiling limit for sodium hydroxide, based on the ACGIH- and NIOSH-recommended limits.
What is the name of Sn3(PO4)4 under the Stock system of nomenclature?
Answers
Answer:
Tin (IV) Phosphate
Explanation:
"Stock nomenclature for inorganic compounds is a widely used system of chemical nomenclature developed by the German chemist Alfred Stock and first published in 1919. In the "Stock system", the oxidation states of some or all of the elements in a compound are indicated in parentheses by Roman numerals."
how might rising atmospheric co2 concentrations lower the ph of the oceans?
Answers
Explanation:
As CO2 concentrations rise, excess CO2 is absorbed by the oceans. CO2 in the oceans can react chemically with water to form acid. ... The difference in pH units between two acidic solutions is three.
I need the solution of C.

Answers
The maximum volume of carbon dioxide that can be formed at RTP would be 0.48 L.
Stoichiometric problemSodium carbonate reacts with hydrochloric acid according to the following balanced equation:
\(Na_2CO_3 + 2HCl -- > 2NaCl + H_2O + CO_2\)
The mole ratio of the sodium carbonate that reacts to the carbon dioxide that is produced is 1:1. Thus 0.020 mol of sodium carbonate will be equivalent to 0.020 mol of carbon dioxide.
At room temperature and pressure (RTP), 1 mole of gas is equivalent to 24 L of gas.
Thus, 0.020 mol carbon dioxide would be equivalent to:
0.020 x 24 = 0.48 L
In other words, the maximum volume of carbon dioxide that can be produced from the reaction at RTP is 0.48 L.
More on stoichiometric problems can be found here: https://brainly.com/question/23742235
#SPJ1
What is the purpose of the periodic table?
Answers
Answer:
the periodic table is important because it is organized to provide a alot of information about elements and how they relate to one another in one easy-to-use reference. The table can be used to predict the properties of elements, even those that have not yet been discovered.
Explanation:
the content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers
Concentration of Mn in 50 ml of unknown steel solution (95.5*5)/50 = 9.55 ppm Given for 9.55 ppm of Mn gives absorbance 0.248. For absorbance 0.185, the concentration of Mn is Conc (ppm)=(9.55*0.185)/0.248=7.12ppm
The capacity to focus means having the ability to control your attention. It means having command of your attention. It is the capacity to keep one's attention on a single idea without getting sidetracked. The capacity to concentrate while ignoring unrelated thoughts is known as attentional focus. The amount of solute in a specific amount of solution is how concentrated a substance is. Molarity, or the number of moles of solute in one liter of solution, is the standard unit of concentration expression. It is the capacity to keep one's attention on a single thing or idea while blocking out all other irrelevant thoughts, ideas, feelings, and sensations.Concentration enables more effective resource management and problem-solving techniques. Concentration makes it less likely that you will overlook crucial information. You can memorize things more quickly if you maintain your focus.
Learn more about Concentration here:
https://brainly.com/question/10725862
#SPJ4
Which reactant will be used up first if 78.1g of o2 is reacted with 62.4g of c4h10?
A. c4h10
B. o2
C. co2
D. h2o
Answers
Answer:
Reagent O₂ will be consumed first.
Explanation:
The balanced reaction between O₂ and C₄H₁₀ is:
2 C₄H₁₀ + 13 O₂ → 8 CO₂ + 10 H₂O
Then, by reaction stoichiometry, the following amounts of reactants and products participate in the reaction:
C₄H₁₀: 2 molesO₂: 13 moles CO₂: 8 moles H₂O: 10 molesBeing:
C: 12 g/moleH: 1 g/moleO: 16 g/moleThe molar mass of the compounds that participate in the reaction is:
C₄H₁₀: 4*12 g/mole + 10*1 g/mole= 58 g/moleO₂: 2*16 g/mole= 32 g/moleCO₂: 12 g/mole + 2*16 g/mole= 44 g/moleH₂O: 2*1 g/mole + 16 g/mole= 18 g/moleThen, by reaction stoichiometry, the following mass quantities of reactants and products participate in the reaction:
C₄H₁₀: 2 moles* 58 g/mole= 116 gO₂: 13 moles* 32 g/mole= 416 gCO₂: 8 moles* 44 g/mole= 352 gH₂O: 10 moles* 18 g/mole= 180 gIf 78.1 g of O₂ react, it is possible to apply the following rule of three: if by stoichiometry 416 g of O₂ react with 116 g of C₄H₁₀, 62.4 g of C₄H₁₀ with how much mass of O₂ do they react?
\(mass of O_{2} =\frac{416grams of O_{2}*62.4 grams ofC_{4}H_{10} }{116 grams of C_{4}H_{10}}\)
mass of O₂= 223.78 grams
But 21.78 grams of O₂ are not available, 78.1 grams are available. Since you have less mass than you need to react with 62.4 g of C₄H₁₀, reagent O₂ will be consumed first.
When an atom becomes a negative ion, it
Answers
It becomes a positives atom. Since it loses electrons it has more of a positive charge than a normal atom.
Reset the simulation again. Set the ruler at 50 and the left platform at 20. Don't add any molecules yet. Predict what will happen when you add 30 molecules of substance A. Why do you think this will happen?
Answers
Answer:
The conversion of substance A to substance B will happen extremely slowly because of the very high activation energy that each molecule must obtain to convert.
Explanation:
Plato answer
The conversion of substance A to substance B will happen extremely slowly.
What are molecules?A molecule is two or more atoms connected by chemical bonds, which form the smallest unit of a substance that retains the composition and properties of that substance.
The conversion of substance A to substance B will happen extremely slowly because of the very high activation energy that each molecule must obtain to convert.
Learn more about the molecules here:
https://brainly.com/question/19922822
#SPJ2
The name for TiSO5 is
Answers
Answer:
Titanium oxide sulphate
Explanation:
The electron configuration of nitrogen (N) is
1s²2s²2p³
1s 2s 2p
1s²2s²2p5
1s²2s²2p
Answers
Answer:
The electron configuration of nitrogen (N) is 1s²2s²2p³ . The electron configuration of an atom describes the distribution of electrons among different energy levels and orbitals. The first number in the electron configuration represents the principal energy level, while the letter represents the sublevel (s, p, d, or f). The superscript number represents the number of electrons in that sublevel .
The electron configuration of nitrogen (N) is 1s²2s²2p³. The correct option is 1.
An atom's electron configuration specifies how its electrons are dispersed throughout the various energy levels and orbitals.
The electron configuration of nitrogen (N), which has an atomic number of 7, is 1s²2s²2p³.
Nitrogen has a total of 7 electrons in this arrangement. The first energy level (1s) is completely occupied by two electrons, and the second energy level (2s) is also completely occupied by two electrons.
The remaining three electrons are in the 2p orbital. The 2p orbital is broken down into three suborbitals: 2px, 2py, and 2pz. Each suborbital may carry a maximum of two electrons, accounting for the three electrons in nitrogen's 2p³ structure.
Thus, the correct option is 1.
For more details regarding electronic configuration, visit:
https://brainly.com/question/29184975
#SPJ5
Your question seems incomplete, the probable complete question is:
The electron configuration of nitrogen (N) is
1s²2s²2p³1s 2s 2p1s²2s²2p51s²2s²2pWhat is the formula mass (molar mass) of cholesterol (C27H46O), a fat found in your blood and cell membranes? 19 g/mol 130 g/mol 260 g/mol 386 g/mol.
Answers
Answer:
386.66 g/mol
Explanation:
To calculate the molar mass of a molecule, you add up the molar masses of its atoms.
Thus, for cholesterol. we get
27C = 27 × 12.011 = 324.297 g
46H = 46 × 1.008 = 46.368
1O = 1 × 15.999 = 15.999
TOTAL = 386.655 g
To two decimal places, the molar mass of cholesterol is 386.66 g/mol