the pKa of diacetamide is ?
Answers
The pKa of diacetamide is approximately 10.5. This means that at a pH below 10.5, diacetamide will exist predominantly in its protonated form, while at a pH above 10.5, it will exist predominantly in its deprotonated form.
The pKa value of a compound is a measure of its acidity, specifically the negative logarithm of the acid dissociation constant (Ka). The pKa value indicates the acidity or basicity of a molecule, and specifically refers to the pH at which the molecule is 50% protonated and 50% deprotonated.
In the case of diacetamide, it has an amide group (-CONH2) which is weakly basic and can accept a proton to form the protonated form of the molecule. Understanding the pKa of diacetamide is important in predicting its behavior in different environments, such as in chemical reactions or in biological systems.
Learn more about dissociation constant here:
brainly.com/question/31607191
#SPJ11
Related Questions
Question 32 convert 0.23 moles h2o to number of molecules. o 3.8 x 10-25 o 0.0039 o 1.39 x 1023 o 13.4 question 33 what are the products in the single displacement reaction of ca and hci. o cacl, h2 o cazci, h2 o cacl2, h2 o capci, h2 question 34 what are the products in the double displacement reaction, na2so4 + nh4ci -->o nacl, (nh4)2so4 o na2ci, (nh4)2so4 o nh4n, ciso4 o co2, h20 click save and submit to save and submit. click save all answers to save all answers 3623
Answers
A) The number of H₂O molecules in 0.23 moles is equal to 1.5 × 10²³.
B) The single displacement reaction is,
Ca + 2HCl → CaCl₂ + H₂
C) The double displacement reaction is,
Na₂SO₄ + 2NH₄Cl → 2NaCl + (NH₄)₂SO₄
Displacement reaction: What is it?A displacement reaction is one in which a set of atoms in a molecule are replaced by another set of atoms.
When an element leaves its compound or when one element is replaced by another from its own compound, a single displacement reaction can be demonstrated as a type of redox reaction.
In aqueous solutions, double displacement reactions take place where ions precipitate and exchange ions.
Hydrogen gas and calcium chloride are produced in a single displacement reaction between calcium and HCl acid.
Ca + 2HCl → CaCl₂ + H₂
To know more about displacement reaction visit:
brainly.com/question/29307794
#SPJ4
what does it mean to say that an enzyme-catalyzed reaction is either enzyme-limited or substrate-limited?
Answers
Nothing changes; it stays the same.
What does it imply to claim that a reaction is being catalysed by an enzyme?
Enzymatic catalysis of a reaction involving two substrates. The two substrates are brought together in the correct direction and location to react with one another using the template provided by the enzyme.
How do enzymes decide what to eat for fuel?
Finding the peptide sequences that proteases cleave in vitro—or, more specifically, which amino acids span the cleavage site and are recognised by the enzyme's active site—is one method of identifying prospective protease substrates. The proteome is then searched for substrates using these sequences, much like partial licence plate numbers
To know more about Enzyme
Visit:
https://brainly.com/question/1996362
#SPJ4
what are the factors affecting gravity?
Answers
Gravity, as a fundamental force of nature, is influenced by several factors. The following are some of the key factors affecting gravity:
Mass: The most significant factor affecting gravity is the mass of the objects involved. According to Newton's law of universal gravitation, the gravitational force between two objects is directly proportional to the product of their masses. Greater mass leads to a stronger gravitational force.Distance: The distance between two objects also plays a crucial role in the strength of gravity. According to the inverse square law, the gravitational force decreases as the distance between objects increases. As objects move farther apart, the gravitational attraction between them weakens.Gravitational Constant: The gravitational constant, denoted by G, is a fundamental constant in physics that determines the strength of the gravitational force. It is a universal constant and does not change, affecting the overall magnitude of gravity.Shape and Distribution of Mass: The distribution of mass within an object can influence the gravitational field it generates. Objects with a more compact and concentrated mass distribution will have a stronger gravitational pull compared to those with a more spread-out mass distribution.External Influences: Gravity can be influenced by external factors such as nearby celestial bodies or the presence of other forces. For example, the gravitational interaction between the Earth and the Moon affects tides on Earth's surface.Calcium hypochlorite, Ca(OCl)2, is a bleaching agent produced from sodium hydroxide, calcium hydroxide, and chlorine. Socium chloride and water are also produced in the reaction. What is the missing coefficient that will balance the chemical equation? ___NaOH + Ca(OH)2 + 2Cl2 → Ca(OCl)2 + 2NaCl + 2H2O a.1 b.4 c.2 d.3
Answers
Answer: The missing coefficient is 2.
Explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
\(2NaOH+Ca(OH)_2+2Cl_2\rightarrow Ca(OCl)_2+2NaCl+2H_2O\)
As in the products, there are 2 atoms of sodium, thus there will be 2 atoms of of sodium in the reactant as well. This will balance the number of hydrogen and oxygen atoms as well.
Thus the missing coefficient is 2.
a serving of cheese its releases 130kcal when digested. if this energy was transferred to 2.5kg of water at 27 degrees celsius, what would the final temperature be?
Answers
The final temperature : 79.07°C
Further explanationHeat transferred can be formulated
Q=m.c.ΔT
Q= heat, J
m=mass,g
c=specific heat, for water 4.18 J/g C
ΔT = temperature
Heat transferred : 130 kcal
1 kcal = 4186 J
130 kcal =
\(\tt 130\times 4186=544180~J\)
mass = 2.5 kg =2500 g
t₁=27 °c
The final temperature :
\(\tt 544180=2500\times 4.18\times (t_2-27)\\\\t_2-27=52.07\\\\t_2=79.07^oC\)
Arsenic, hydraulic fracturing, lead, and PFAS present chemical threats to global drinking water supplies in different ways. For each problem, describe: (a) the origin of exposure, (b) human health consequences, (c) drivers of continued exposure, and (d) examples of modern solutions.
Answers
Arsenic, hydraulic fracturing, lead, and PFAS present chemical threats to global drinking water supplies in different ways.
Let's discuss each of them in detail:
(a) Arsenic - The origin of arsenic exposure is natural deposits or contamination from agricultural or industrial practices. Human health consequences include skin, lung, liver, and bladder cancers. It can also lead to cardiovascular diseases, skin lesions, and neurodevelopmental effects. Drivers of continued exposure include poor regulation and monitoring. Modern solutions include rainwater harvesting and treatment.
(b) Hydraulic fracturing - Hydraulic fracturing involves using a mixture of chemicals, water, and sand to extract natural gas and oil from shale rock formations. The origin of exposure is contaminated surface and groundwater due to the release of chemicals from fracking fluids and other sources. Human health consequences include skin, eye, and respiratory irritation, headaches, dizziness, and reproductive and developmental problems. Drivers of continued exposure include lack of regulation and poor oversight. Modern solutions include alternative energy sources and regulation of the industry.
(c) Lead - Lead contamination in drinking water can occur due to corrosion of plumbing materials. Human health consequences include neurological damage, developmental delays, anemia, and hypertension. Drivers of continued exposure include aging infrastructure and poor maintenance. Modern solutions include replacing lead service lines, testing for lead levels, and implementing corrosion control.
(d) PFAS - PFAS (per- and polyfluoroalkyl substances) are human-made chemicals used in a variety of consumer and industrial products. They can enter the water supply through wastewater discharges, firefighting foams, and other sources. Human health consequences include developmental effects, immune system damage, cancer, and thyroid hormone disruption. Drivers of continued exposure include the continued use of PFAS in consumer and industrial products. Modern solutions include reducing the use of PFAS in products and treatment methods such as granular activated carbon.
To know more about hydraulic fracturing visit:
https://brainly.com/question/31032804
#SPJ11
starting with known concentrations of x and y in experiment 1, the rate of formation of z was measured. if the reaction was first order with respect to x and second order with respect to y, the initial rate of formation of z in experiment 2 would be
Answers
If the reaction is first order with respect to x and second order with respect to y, the rate law can be written as rate = k[X]⁻¹[Y]⁻².
Starting with known concentrations of x and y in experiment 1, the initial rate of formation of z was measured. The initial rate of formation of z in experiment 2 would depend on the new concentrations of x and y. If the new concentrations are different from the initial concentrations used in experiment 1, the initial rate of formation of z in experiment 2 will also be different.
To determine the initial rate of formation of z in experiment 2, the rate law equation would need to be used with the new concentrations of x and y. The units of k, the rate constant, determine the units of the rate.
To know more about first order here
https://brainly.com/question/13329678
#SPJ4
--The given question is incomplete, the complete question is
"738; (1,45)(0.042 T Jath) 8518,314(308) 4 OO (3.O0)L85) Ttn (0.0821)(35.0) Enic snele Initial Rate of (Ylo Formation of (moLL- sec 0.101 9l 0.20 Experiment (Xlo 0.40 0.20 The table above shows the results from rate study of the reaction X+Y 2. Starting with known concentrations of X and Y in experiment 1,the rate of formation of Z was measured. If the reaction was first order with respect to( X and second order with respect t0_Y! the initial rate of formation of 2 experiment 2 would be (A) R (B) 2 Cx]" [Y]' 2R Y 1 = 2 1 4R."--
select all of the following that are produced by one round of the krebs cycle.
A. ATP
B. NADH
C. FADH2
D. CO2
Answers
One round of the Krebs cycle produces (B) NADH (C) FADH₂ (D) CO₂. Hence, the correct options are B,C and D.
NADH and FADH2 are electron carriers that will go on to produce ATP in the electron transport chain. CO2 is a waste product that is released into the atmosphere. ATP is not directly produced by the Krebs cycle, but rather by the electron transport chain, which uses the NADH and FADH2 produced by the Krebs cycle to generate ATP. The Krebs cycle, also known as the citric acid cycle or the tricarboxylic acid cycle, is a series of biochemical reactions that occur in the mitochondria of eukaryotic cells, as well as in the cytoplasm of prokaryotic cells. The purpose of the Krebs cycle is to generate energy in the form of ATP, as well as to produce electron carriers such as NADH and FADH2 that will go on to generate more ATP in the electron transport chain. In the Krebs cycle, acetyl-CoA, a two-carbon molecule derived from the breakdown of carbohydrates, lipids, and proteins, enters the cycle and is combined with a four-carbon molecule called oxaloacetate to form citrate, a six-carbon molecule. Citrate is then converted through a series of reactions into isocitrate, which is then converted into alpha-ketoglutarate. During these reactions, CO2 is released as a waste product. Alpha-ketoglutarate is then converted into succinyl-CoA, which releases another molecule of CO2. This reaction produces a molecule of ATP as well as a molecule of the electron carrier NADH. Succinyl-CoA is then converted into succinate, which is further converted into fumarate, releasing another molecule of FADH2. Fumarate is then converted into malate, which is then converted back into oxaloacetate, which can combine with another molecule of acetyl-CoA to continue the cycle.
To know more about Krebs cycle please refer: https://brainly.com/question/13153590
#SPJ4
At which location would an object’s weight be the greatest?
on Pluto
on Earth
on the Sun
on the moon
Answers
Answer:
the sun
Explanation:
because its the center
The object's weight would be the greatest:
C. on the sun, this can be accounted on the Earth's gravity
Object's weight at the sun:At 12 PM, when the Sun is straightforwardly beneath, it pulls on an item in a similar course as the draw of the Earth; around early afternoon, when the Sun is straight above, it pulls on an article toward a path inverse to the draw of the Earth.
Thus, all articles ought to be heavier around evening time than they are at the day. Sun's gravitational draw on earth-bound objects is around 0.0006 of earth's gravity.
Hence, option c is correct.
Find more information about Gravitational pull here:
brainly.com/question/174980
Which events are examples of weathering or erosion from water? I dont know if its chemistry.
Waves pound the rocks of an ocean beach.
Waves pound the rocks of an ocean beach.
A river carries small rocks down a mountain.
A river carries small rocks down a mountain.
A marsh becomes drier as its water evaporates.
A marsh becomes drier as its water evaporates.
Clouds form from water vapor condensing in the air.
Clouds form from water vapor condensing in the air.
Underground water dissolves limestone, forming a cave.
Answers
Examples of erosion or corrosion caused by water include: An coastal beach's rocks are battered by the waves. Small rocks are carried down a hillside by a river. A tunnel is created when underground water dissolves limestone.
What kind of circumstance exemplifies weathering?Rust, which results from oxidation, and acid rain, which is brought on when carbonic acid dissolves rocks, are two instances of chemical weathering. additional molecular weathering.
What kind of degradation is caused by weathering?On a mountainside, tiny rock fragments break off due to wind and precipitation. Chemical and mechanical mechanisms can cause weathering. The migration of particles away from their source is erosion. A tiny rock fragment is carried away from a mountainside by the wind as an illustration of erosion.
To know more about corrosion visit:-
https://brainly.com/question/30057568
#SPJ1
If you burn a piece of wood, it becomes a different substance. What type of
property is this?
A. Electrical property
O
B. Chemical property
C. Physical property
D. Mechanical property
Answers
Answer:
B. Chemical property
Explanation:
Answer:
B
Explanation:
a reaction has taken place in which you can't get back what you started with
SO2+02➡SO3
balance chemical equation
Answers
Answer:
2S02 + O2 ---> 2SO3
Its balanced now as Reactants = Products.
How many Cl- bond with one Na+ ion?
Answers
Hope this helped
WILL GIVE BRALINLIST
The solution to 1.56 x 65.3211 should have ____ significant figures.
Answers
Answer: 3 Figures
Explanation:
Calculate 345.009 g - 23.009 g and give your answer with the appropriate number of significant figures. 322.000 g.
there bud
Is heat a form of kinetic energy or potential energy?
Answers
According to the graph,
what is the ratio of
reactants and products
when the reaction rate
stops changing or it
reaches equilibrium?
Answers
When a chemical reaction reaches equilibrium, the concentration graph will typically show no further changes
What happens to the concentration graph when a reaction reaches equilibrium?This question is incomplete but it appears you want to know something about the concentration graph of a reaction at equilibrium.
When a chemical reaction reaches equilibrium, the concentration graph will typically show no further changes in the concentrations of the reactants and products over time. This means that the graph will become relatively flat and stable after a certain point, indicating that the reaction has reached a state of dynamic balance where the rate of the forward reaction is equal to the rate of the reverse reaction.
At equilibrium, the concentrations of the reactants and products are constant, but they may not necessarily be equal. The specific concentrations of each species at equilibrium depend on the particular reaction and the conditions under which it is carried out (such as temperature, pressure, and concentration of reactants and products).
Learn more about reaction equilibrium:https://brainly.com/question/15118952
#SPJ1
Answer:
there are more products than reactants
Explanation:
acellus confirmed
Calculate ΔH for 2 NO(g) + O2(g) → N2O4(g) using the following information: N2O4(g)2 NO(g) + O2(g)→→2 NO2(g)2 NO2(g)ΔHΔH==+57.9 kJ−113.1 kJ Calculate for using the following information: 2.7 kJ -55.2 kJ -85.5 kJ -171.0 kJ +55.2 kJ
Answers
The heat of reaction, or ΔH, is also known as the enthalpy of reaction. The enthalpy for heat for the equation is ΔH=9.2 KJ.
N2 + O2 + 2NO H=180.6 KJ/mol 2NO2 + N2O4 H=-57 KJ/mol
2NO + O22NO2 H= -114.4 (note the sign change to N2+2O2N2O4 H= 9.2 KJ).
We may obtain the O2 on the left side, or the (reactant side), by inverting the third equation, allowing us to obtain a total of 2O2 in the bottom-most final equation. Remove 2NO2 from the reactant side of equation 1 and the product side of equation 2 both. Additionally, you should delete 2NO from the third equation's reactant and product sides. This results in an identical situation above and below the line.
Since equation 3 must be reversed for the change in enthalpy to remain the same, you must enter -57, 180.6, and -114.4 to obtain 9.2 KJ.
To know more about enthalpy of heat
https://brainly.com/question/16980891
#SPJ4
The complete question is
Use Hess's law and the following equations to calculate ΔH for the reaction N2(g) + 2O2(g) N2O4(g). (Show your work.) (8 points)
2NO2(g) N2O4(g) ΔH = –57.0 kJ/mol
N2(g) + O2(g) 2NO(g) ΔH = 180.6 kJ/mol
2NO2(g) 2NO(g) + O2(g) ΔH = 114.4 kJ/mol
a) Based on the functional groups shown, what type of molecule is this ______________________
b) What are the monomers of this macromolecule called? ______________________
c) What is the name of the bond that exists between the monomers ____________________
d) This molecule can have ___________ levels of structure
e) What level of structure is shown in the picture? Why do you think so? _______________________
f)If I add another chain to this molecule what level of structure will that be?__________________
g) What are the other levels of structure can it have and how are they formed?
Answers
a) Based on the functional groups shown, the molecule appears to be a protein.
b) The monomers of proteins are called amino acids.
c) The bond that exists between the monomers of proteins is called a peptide bond.
d) Proteins can have four levels of structure: primary, secondary, tertiary, and quaternary.
e) The level of structure shown in the picture is difficult to determine without a clear image or additional information. However, based on the general representation of proteins, it is likely depicting the secondary structure, specifically an alpha helix or beta sheet.
f) If another chain is added to the molecule, it would result in the formation of the quaternary structure.
g) Proteins can have various levels of structure. The primary structure refers to the linear sequence of amino acids. The secondary structure includes the folding of the protein into patterns like alpha helices and beta sheets.
a) To determine the type of molecule based on functional groups, it would be helpful to describe or provide the functional groups present in the image. Different functional groups are characteristic of different macromolecules.
For example, amino and carboxyl groups are characteristic of proteins, hydroxyl groups are characteristic of carbohydrates, and carboxyl and methyl groups are characteristic of lipids. Please describe the functional groups you see in the image to help identify the molecule accurately.
b) Once the functional groups are identified, the monomers of the corresponding macromolecule can be determined. For instance, proteins are composed of amino acids, carbohydrates are composed of monosaccharides, and lipids can be composed of fatty acids or glycerol molecules.
c) The bond that exists between monomers in proteins is called a peptide bond, which forms through a condensation reaction between the amino group of one amino acid and the carboxyl group of another amino acid.
d) Proteins exhibit four levels of structure: primary, secondary, tertiary, and quaternary. Each level of structure describes different aspects of protein folding, organization, and interactions.
e) Without specific information about the image, it is challenging to determine the exact level of protein structure shown. However, common representations of proteins often depict the secondary structure, such as alpha helices or beta sheets, which are formed through hydrogen bonding between the amino acid backbone.
f) If another chain is added to the protein molecule, it would result in the formation of the quaternary structure. The quaternary structure arises when multiple protein subunits come together to form a functional protein complex.
g) Proteins can have additional levels of structure. The primary structure refers to the linear sequence of amino acids, while the secondary structure includes local folding patterns. The tertiary structure involves the overall three-dimensional folding of the protein, influenced by interactions between amino acid side chains.
These interactions include hydrogen bonding, hydrophobic interactions, disulfide bonds, and more. The quaternary structure arises from the arrangement of multiple protein subunits and the interactions between them.
Learn more about functional groups here:
https://brainly.com/question/1356508
#SPJ11
what is ATP ????????
Answers
Answer:
If youre talking about biology its source of energy that is storage in the cellular level.
Explanation:
Calculate the molarity of a solution that contains 0.75 moles of lithium fluoride, LiF, in a 65 mL solution.
Answers

The molarity (M) of a solution is 11.5 M that contains 0.75 moles of lithium fluoride, LiF in a 65 ml of solution.
What is Molarity ?Molarity (M) is defined as the number of moles of solute dissolved in 1L of solution. Molarity is also known as Molar concentration. The S.I Unit of Molarity is molar (M) or mol/L.
How to find molarity of a solution ?Molarity (M) = \(\frac{\text{Number of moles of solute}}{\text{Volume of solution (in liters)}}\)
From the above definition it is clear that the volume of solution is in liter. In question the volume of solution is given in ml.
So, convert 65 ml into l.
65 ml = \(\frac{65}{1000}\) = 0.065 l
Now, put the values in above formula, we get that
(M) = \(\frac{0.75}{0.065}\) = 11.5 M
Thus, the molarity of a solution is 11.5 M that contains 0.75 moles of lithium fluoride, LiF in a 65 ml of solution.
Learn more about Molarity here: https://brainly.com/question/14469428
#SPJ2
what is the bond order of the c‒c bond in acetylene (ethyne, c2h2)?
Answers
The bond order of the C‒C bond in acetylene (ethyne, C₂H₂) is 2, a triple bond.
To determine the bond order, we need to examine the bonding between the carbon atoms in acetylene. Acetylene consists of two carbon atoms, each bonded to a single hydrogen atom, and connected by a triple bond.
In a Lewis structure representation, we can depict the C‒C bond in acetylene as a triple bond, consisting of one sigma (σ) bond and two pi (π) bonds. The sigma bond is formed by the overlap of hybridized orbitals on the carbon atoms, while the two pi bonds are formed by the overlap of unhybridized p orbitals.
The bond order is a measure of the number of electron pairs shared between two atoms in a molecule. For acetylene, the bond order is calculated by taking the difference between the number of bonding electrons and the number of antibonding electrons and dividing it by two.
In the case of the C‒C bond in acetylene, we have one sigma bond and two pi bonds. Each bond consists of two electrons, so we have a total of 4 bonding electrons. There are no antibonding electrons in the C‒C bond. Therefore, the bond order is:
Bond Order = (Number of Bonding Electrons - Number of Antibonding Electrons) / 2
= (4 - 0) / 2
= 2
Hence, the bond order of the C‒C bond in acetylene (ethyne, C₂H₂) is 2, indicating a triple bond. The presence of a triple bond makes the C‒C bond in acetylene shorter and stronger compared to a single or double bond.
To know more about bond order, refer to the link below:
https://brainly.com/question/12447843#
#SPJ11
How many milliliters of a 5.0 M H2SO4 stock solution would you need to prepare 100.0 mL of 0.25 M H2SO4?
Answers
Answer:
5 milliliters
Explanation:
Use the formula M1V1 = M2V2 where M is molarity and V is volume
Plug in numbers
(.25 M)(100 mL) = (5 M)V2
V2 = 5 mL
The volume of stock solution of 5M H₂SO₄ needed to prepare 100mL of 0.25M H₂SO₄ is 5mL.
How do we calculate the volume?Volume of stock solution to prepare any dilute solution will be calculated by using the following formula:
M₁V₁ = M₂V₂, where
M₁ & V₁ is the molarity and volume of stock solution, and
M₂ & V₂ is the molarity and volume of final prepared solution.
On putting values from the question to the above formula and calculate for the value of V₁ as:
V₁ = (0.25)(100) / (5)
V₁ = 5 mL
Hence required volume is 5mL.
To know more about volume & molarity, visit the below link:
https://brainly.com/question/24881505
Would a rollercoaster have the greatest kinetic energy at the top of the highest hill or at the bottom on the highest hill
Answers
Answer:
The rollercoaster has the lowest kinetic energy at the top of the hill.
The rollercaoster has the highest kinetic energy at the bottom of the hill.
Explanation:
The kinetic energy of any solid body in motion is usually computed using this formula:
K.E = \(\frac{1}{2}mv^2\)
From this, we can see that it varies based on two major parameters - The mass of the moving object and the velocity of the moving object.
We can assume that the mass of the rollercoaster is constant since no one gets off and it does not shrink in its size during the ride.
This means that the variations in the K.E are mainly coming from its velocity.
At the top of the hill, the rollercoaster is moving at its slowest pace. hence, it has the lowest kinetic energy at the top of the hill.
However, at the bottom of the hill, the rollercoaster is moving its fastest, hence it has the highest kinetic energy at the bottom of the hill.
PLEASE HELP!
What is the composition of the sigma bonds in methane i.e. what atomic orbits comprise the molecular orbit? Hint: they are all the same, so there is only one kind sigma bond here.
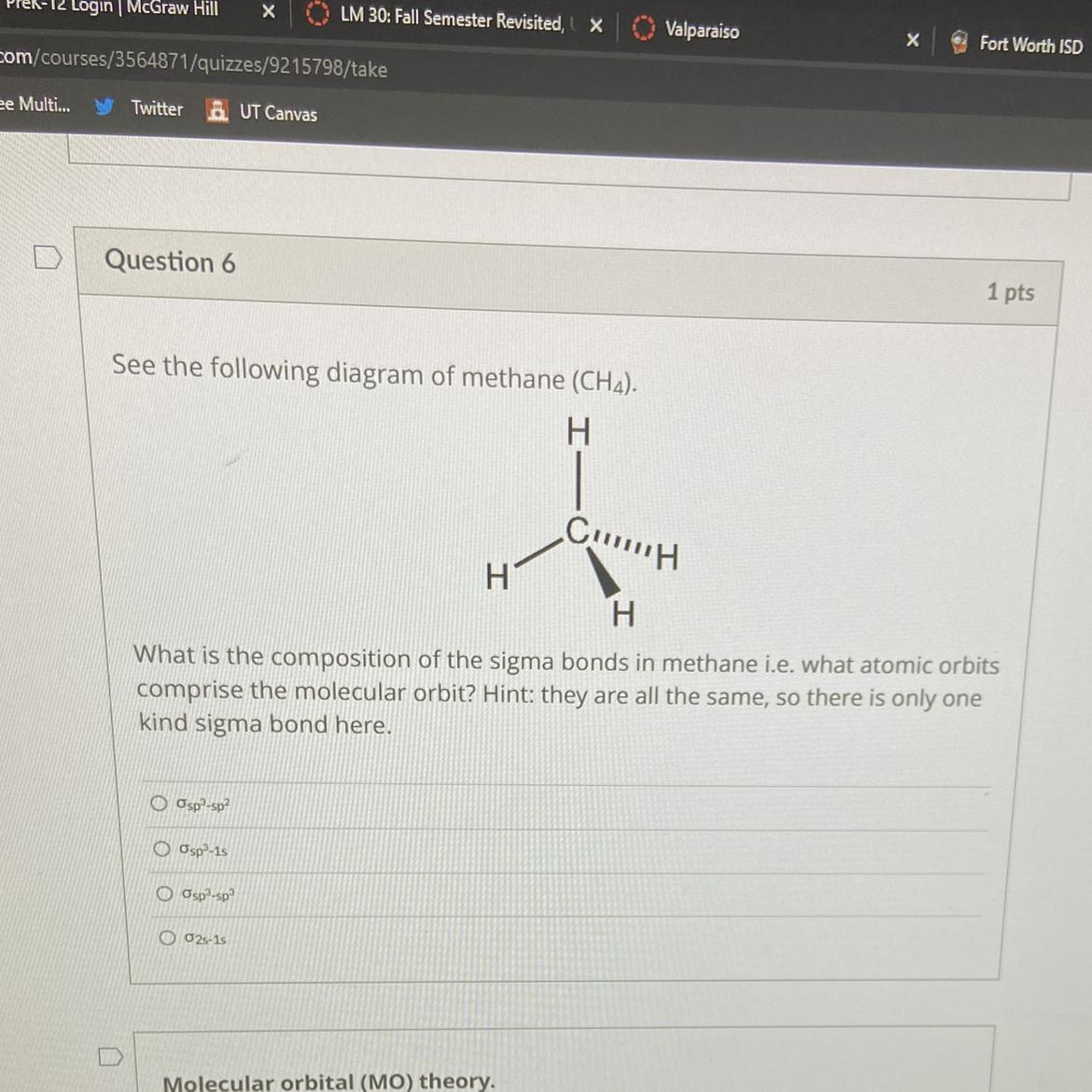
Answers
The 1s-orbital from the a hydrogen to either a sigma bond forms a link with one of the four sp3 hybrid orbitals of carbon.
What has a methane destructor?Methane is naturally eliminated by a variety of biological and chemical processes, including the reaction of methane-eating microorganisms (methanotrophs) on soil and water with air hydroxyl [OH] and chlorine.
How is methane dangerous to people?The quantity of oxygen absorbed from the air might be decreased by high methane levels. This may include headaches, facial flushes, slurred speech, visual issues, memory loss, changes in mood, and vision abnormalities. In severe situations, respiration and heart rate fluctuations, balance issues, numbness, and unconsciousness may occur.
To know more about methane visit:
https://brainly.com/question/2127750
#SPJ1
Bombardier beetles release a burst of hot chemical spray with a popping sound. The chemical is sprayed from a structure present under the belly of the beetles.
Which is the likely advantage of such a behavior?
It allows the beetles to attract mates.
It allows the beetles to move obstacles out of their paths.
It allows the beetles to escape from predators.
It allows the beetles to stay warm in cold conditions.
Answers
It allows the beetles to escape from predators
an alloy is a mixture of elements that has metallic properties.
Answers
True. An alloy is a solid solution consisting of two or more metallic elements, or a metallic element and a non-metallic element.
The properties of an alloy are usually different from those of its constituent elements. The reason for this is that the atoms of different elements in an alloy interact with each other differently than they would if they were in their pure elemental form. This interaction can lead to changes in the electronic and crystal structure of the alloy, which in turn can affect its physical and chemical properties. Some common examples of alloys include brass (copper and zinc), stainless steel (iron, chromium, and nickel), and bronze (copper and tin).
Learn more about alloys here: brainly.com/question/30432755
#SPJ4
Complete question:
An alloy is a mixture of elements that has metallic properties. True or False
A gas mixture with a total pressure of 4.6 atm is used in a hospital. If the mixture contains 5.4 mol of nitrogen and 1.4 mol of oxygen, what is the partial pressure, in atmospheres, of each gas in the sample?
Answers
Considering the Dalton's partial pressure, the partial pressure of nitrogen and oxygen is 3.634 atm and 0.966 atm respectively.
The pressure exerted by a particular gas in a mixture is known as its partial pressure.
So, Dalton's law states that the total pressure of a gas mixture is equal to the sum of the pressures that each gas would exert if it were alone:
\(P_{T} =P_{1} +P_{2} +...+P_{n}\)
where n is the amount of gases.
Dalton's partial pressure law can also be expressed in terms of the mole fraction of the gas in the mixture.
The mole fraction is a dimensionless quantity that expresses the ratio of the number of moles of a component to the number of moles of all the components present:
\(x_{A} =\frac{n_{A} }{n_{total} }\)
So in a mixture of two or more gases, the partial pressure of gas A can be expressed as:
\(P_{A} =x_{A} P_{T}\)
In this case, the mixture contains 5.4 mol of nitrogen and 1.4 mol of oxygen. So, the total number of moles of all the components present is 5.4 moles + 1.4 moles= 6.8 moles.
Then, the mole fraction of each gas can be calculated as:
\(x_{nitrogen} =\frac{n_{nitrogen} }{n_{total} }=\frac{5.4 moles}{6.8 moles} = 0.79\)\(x_{oxygen} =\frac{n_{oxygen} }{n_{total} }=\frac{1.4 moles}{6.8 moles} = 0.21\)So, being 4.6 atm the total pressure of the gas mixture, the partial pressure of each gas can be calculated as:
\(P_{nitrogen} =x_{nitrogen} P_{T}\)= 0.79× 4.6 atm= 3.634 atm
\(P_{oxygen} =x_{oxygen} P_{T}\)= 0.21× 4.6 atm= 0.966 atm
In summary, the partial pressure of nitrogen and oxygen is 3.634 atm and 0.966 atm respectively.
Learn more:
brainly.com/question/14239096?referrer=searchResults brainly.com/question/25181467?referrer=searchResults brainly.com/question/14119417what is the formula unit for a compound made from Li and Cl?
Answers
LiCl
because Li loses 1 electron ==> Li+
and Cl gains 1 electron ==> Cl-
Li+ Cl-
we use the criss cross rule
===> LiCl
Which bond is a very strong dipole-dipole force?
A. A covalent bond
B. An ionic bond
C. A hydrogen bond
D. A metallic bond
Answers
Answer : C. A hydrogen bond
Explanation:
Answer:
A. covalent bond
Explanation:
dipole-dipole interaction results from difference in electronegativity , eg H-F where Fluorine has strong electronegativity but Hydrogen has less electronegativity. hence the bond has dipole moment towards F atom.
Calculate the Empirical Formula for the following compound:
0.300 mol of S and 0.900 mole of O.
Answers
Answer:
\(\boxed {\boxed {\sf SO_3}}\)
Explanation:
An empirical formula shows the smallest whole-number ratio of the atoms in a compound.
So, we must calculate this ratio. Since we are given the amounts of the elements in moles, we can do this in just 2 steps.
1. DivideThe first step is division. We divide the amount of moles for both elements by the smallest amount of moles.
There are 0.300 moles of sulfur and 0.900 moles of oxygen. 0.300 is smaller, so we divide both amounts by 0.300
Sulfur: 0.300/0.300= 1 Oxygen: 0.900/0.300= 3 2. Write Empirical FormulaThe next step is writing the formula. We use the numbers we just found as the subscripts. These numbers go after the element's symbol in the formula. Remember sulfur is S and there is 1 mole and oxygen is O and there are 3 moles.
S₁O₃This formula is technically correct, but we typically remove subscripts of 1 because no subscript implies 1 representative unit.
SO₃\(\bold {The \ empirical \ formula \ for \ the \ compound \ is \ SO_3}}\)