The percentage of the X-21 isotope is 87.99 % What is the contribution (in amu) to the weighted average from the X-21 isotope, which has a mass of 21.00 amu
Answers
\((21.00)(0.8799) \approx \boxed{18.48 \text{ amu (to 4 sf)}}\)
Related Questions
Of the four gases shown in the chart, which do you think contributes the most to the greenhouse effect? Cite data from the chart to support your answer.

Answers
Greenhouse gases are the gases that trap heat in the atmosphere of the earth. Methane is the greenhouse gas that contributes most to the greenhouse effect.
What is the greenhouse effect?The greenhouse effect is the process due to the greenhouse gas that entraps the heat and sun rays from the sun that increases the temperature of the atmosphere.
The methane gas is found as 180th million in 1 percent and lives around 25 years in the atmosphere also has 25 more strength than carbon dioxide. Methane is found as natural gas and is a potent greenhouse gas.
Therefore, methane contributes most to the greenhouse effect.
Learn more about the greenhouse effect here:
https://brainly.com/question/770740
#SPJ1
Phosphoric acid (H3PO4) in water solution at 25 °C with a concentration of 0. 21 kg mol/m³ is passing through a porous ceramic filter. The thickness of the filter is 4. 2 mm, and its tortuosity numerical value is 13 times the numerical value of its void fraction. The mass transfer rate was estimated to be 1. 4 x 10-9 kg mol H3PO4/ s. M².
a) Predict the diffusion coefficient of Phosphoric acid in water using the Wilke-Chang method.
[CO1, PO1,C4]
b) Calculate the Phosphoric acid concentration at the other side of the ceramic filter.
[CO1, PO1, C4]
Answers
The Wilke-Chang method is used to predict the diffusion coefficient of Phosphoric acid in water. The Wilke-Chang method is a widely used empirical equation to estimate the diffusion coefficient of a solute in a solvent.
Fick's Law of diffusion states that the mass transfer rate of a solute across a porous membrane is proportional to the concentration gradient and the diffusion coefficient.
a. It takes into account the molecular weight, viscosity, and density of the solute and solvent. By plugging in the relevant values for Phosphoric acid and water, we can calculate the diffusion coefficient.
To calculate the diffusion coefficient of Phosphoric acid in water using the Wilke-Chang method, we need to know the molecular weight of Phosphoric acid (H3PO4) and water. The molecular weight of H3PO4 is 98 g/mol, and the molecular weight of water is 18 g/mol.
The Wilke-Chang equation is given by:
D = (1 / Φ) * [(1/M1 + 1/M2) / (√(1/μ1) + √(1/μ2))] * (T / P)
where D is the diffusion coefficient, Φ is the void fraction of the ceramic filter, M1 and M2 are the molecular weights of the solute and solvent, μ1 and μ2 are the viscosities of the solute and solvent, and T is the temperature in Kelvin.
b. We have the mass transfer rate and the thickness of the ceramic filter, so by rearranging Fick's Law equation, we can calculate the concentration at the other side of the filter.
The equation is given by:
J = -D * ∆C/∆x
where J is the mass transfer rate, D is the diffusion coefficient, ∆C is the change in concentration, and ∆x is the thickness of the ceramic filter.
We have the mass transfer rate (1.4 x 10^(-9) kg mol H3PO4/s.m²) and the thickness of the ceramic filter (4.2 mm = 0.0042 m), so by rearranging the equation, we can calculate the change in concentration (∆C) and then use it to find the concentration at the other side of the ceramic filter.
To know more about diffusion coefficient refer to this:
https://brainly.com/question/33711482
#SPJ11
An empty plastic or glass dish being removed from a microwave oven can be cool to the touch, even when food on an adjoining dish is hot. How is this phenom- enon possible?
Answers
Answer:
See explanation
Explanation:
Microwaves work by flipping water molecules upside down, then right side up again, and so on at very high speeds, and the friction generates heat. Some glass or plastic isn't affected by the microwaves, so the molecules don't get flipped, and so no friction occurs and no heat is produced, leading to the dish not being heated up.
ASAP!!!!!!!! test!! will give brainlist!!

Answers
Answer:
the answer to your question is D. 4.5 seconds hope this helps.
Explanation:
A fixed mass of an ideal gas is heated from 50 to 80°C at a constant volume of (a) 1 m3 and (b) 3 m. For which case do you think the energy required will be greater? Multiple Choice a) The energy required will be greater for the case with a constant volume of 3 m3. b) The energy required will be the same for both the cases. c) The energy required will be greater for the case with a constant volume of 1 m3
Answers
The energy required will be greater for the case with a constant volume of 1 m³. The correct option is c)
According to the First Law of Thermodynamics, the change in internal energy of a system is equal to the heat added to the system minus the work done by the system. Since the volume is constant in both cases, there is no work done by the system. Therefore, the energy required to heat the gas is equal to the heat added to the system.
The heat required to raise the temperature of the gas can be calculated using the specific heat capacity of the gas, the mass of the gas, and the temperature change. Since the specific heat capacity of the gas is constant, the energy required to raise the temperature of the gas will depend only on the mass of the gas and the temperature change.
The mass of the gas is constant in this case. Thus, the energy required to heat the gas is directly proportional to the temperature change. Since the temperature change is greater in the case with a constant volume of 1 m³ (30°C) than in the case with a constant volume of 3 m³ (30/3=10°C), the energy required will be greater for the case with a constant volume of 1 m³.
To know more about First Law of Thermodynamics refer here:
https://brainly.com/question/3808473#
#SPJ11
What is the wavelength (in nm) of an electron with the following kinetic energies? (a) 20. 0 ev (no response) nm (b) 200 ev (no response) nm (c) 2. 00 kev (no response) nm (d) 20. 0 kev (no response) nm (e) 0. 200 mev (no response) nm (f) 2. 00 mev (no response) nm which of these energies are most suited for study of the nacl crystal structure? (select all that apply. ) 20. 0 ev 200 ev 2. 00 kev 20. 0 kev 0. 200 mev 2. 00 mev none of these
Answers
A mass of 9.1 1031 kilograms (Kg). A wavelength of 1010m, or around the size of an atom, can be calculated from the de Broglie relation. Due to this, we may directly examine the atomic structure of a crystal using electron microscopes.
Using the de Broglie relation between the momentum p and the wavelength of an electron (=h/p, where h is Planck constant), the wavelength of an electron is computed for a given energy (accelerating voltage). When the wavelength is expressed in meters, 1/ stands for the number of waves in a wave train that can fit within a length of one meter, or, if the wavelength is expressed in centimeters, the number of waves that can fit within a length of one centimeter.
To know more about wavelength ,
https://brainly.com/question/24452579
#SPJ4
what is the net cell reaction for the chromium-silver voltaic cell?
Answers
Cr(s) → Cr3+(aq) + 3e-Ag+(aq) + e- → Ag(s)
To solve such this we must know the concept of chemical reaction. Therefore, the balanced net cell reaction for the chromium-silver voltaic cell is Cr(s)+3Ag⁺(aq)→Cr³⁺(aq)+3Ag(s)
What is chemical reaction?Chemical reaction is a process in which two or more than two molecules collide in right orientation and energy to form a new chemical compound. The mass of the overall reaction should be conserved. There are so many types of chemical reaction reaction like combination reaction, double displacement reaction. Chromium-silver voltaic is a cell that is used in electrochemistry.
The balanced net cell reaction for the chromium-silver voltaic cell is
Cr(s)+3Ag⁺(aq)→Cr³⁺(aq)+3Ag(s)
Therefore, the balanced net cell reaction for the chromium-silver voltaic cell is
Cr(s)+3Ag⁺(aq)→Cr³⁺(aq)+3Ag(s)
Learn more about the chemical reactions, here:
https://brainly.com/question/3461108
#SPJ2
PLEASE ANSWER QUICK 55 POINTS RIGHT ANSWERS ONLY :)

Answers
Explanation:
To solve the problem, we can use the freezing point depression equation:
ΔT = Kf · molality
where ΔT is the change in freezing point, Kf is the freezing point depression constant, and molality is the concentration of the solution in moles of solute per kilogram of solvent.
In this case, we're looking for the freezing point of a solution of C5H4 in benzene, given that the freezing point of pure benzene is 5.50 °C, and the freezing point depression constant is 5.12 °C/m.
First, let's calculate the molality of the solution:
molality = moles of solute / kilograms of solvent
To find the moles of solute, we need to know the molar mass of C5H4. By looking it up in a periodic table, we find:
Molar mass of C5H4 = 64.09 g/mol
The problem doesn't tell us how much solute was added, but it does give us the concentration of the solution as 0.41 m (which means 0.41 moles of C5H4 per kilogram of benzene). Therefore:
molality = 0.41 moles / 0.998 kg ≈ 0.411 mol/kg
Now we can calculate the freezing point depression:
ΔT = Kf · molality
ΔT = 5.12 °C/m · 0.411 mol/kg ≈ 2.10 °C
The freezing point depression tells us how much the freezing point of the solution is lowered compared to the freezing point of pure benzene. Therefore, the freezing point of the solution is:
Freezing point = 5.50 °C - 2.10 °C = 3.40 °C
Therefore, the freezing point of the 0.41 m solution of C5H4 in benzene is 3.40 °C.
suppose the sample of magnesium used in this lab was contaminated with another metal that does not react with hydrochloric acid. how would this have changed your results?
Answers
If the sample of magnesium used in a lab was contaminated with another metal that doesn't react with hydrochloric acid, then the results obtained in the experiment would be affected.
This is because the data collected during the experiment would reflect the reaction between hydrochloric acid and the contaminated sample instead of pure magnesium. As a result, the following changes in results might have been observed:
1. The mass of the contaminated sample would be higher than the mass of pure magnesium.
2. The rate of reaction between the contaminated sample and hydrochloric acid would be slower than the reaction between pure magnesium and hydrochloric acid.
3. The volume of hydrogen gas collected from the reaction would be lower than the volume of hydrogen gas collected in the reaction between pure magnesium and hydrochloric acid.
learn more about contaminated here
https://brainly.com/question/465199
#SPJ11
How does nuclear fusion produce energy in a star?
Answers
Nuclear fusion in stars, such as our Sun, produces energy through the fusion of light atomic nuclei, mainly hydrogen, into heavier nuclei like helium. This fusion process releases a tremendous amount of energy.
Within the core of a star, where temperatures and pressures are extremely high, nuclear fusion takes place. The collisions between hydrogen atoms at such high temperatures provide the necessary energy to overcome electrostatic repulsion, enabling the fusion process.
In the proton-proton chain, the most common fusion process in stars, hydrogen nuclei combine to form helium nuclei through several reactions. The conversion of a small fraction of mass into energy, as described by Einstein's mass-energy equivalence, results in the release of energy in the form of gamma rays.
These high-energy photons interact with matter, gradually transforming into light and heat. This energy release sustains the star's stability by countering gravitational collapse and powers its luminosity for billions of years.
To know more about gamma rays click here: brainly.com/question/14847283
#SPJ11
I need an explanation please

Answers
Endothermic processes are when heat is absorbed and that causes the temperature to drop. Exothermic is when heat is released causing the temperature to rise. Endothermic reactions are always positive and exothermic processes are negative.
Option A
Mark the line thermometer reading decreases.
It means the reaction absorbed heat from surroundingsHence it's endothermicWhat pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
What type of soil has the most permeability
Answers
What minimum temperature is needed to dissolve 60 grams of NH4Cl in 100 g H2O?
Answers
Answer:
At 70∘C , ammonium chloride has a solubility of about 62 g / 100 g H2O . This tells you that can only hope to dissolve 62 g of ammonium chloride for every 100 g of water before the solution becomes saturated.
Which family (group) of elements has a valence-level electron configuration of 1?
a. noble gases
b. halogens
c. alkali metals
d. alkaline earth metals
Answers
The family (group) of elements that have a valence-level electron configuration of 1 are alkali metals. Hence, the answer is option C.
A group or family in the periodic table consists of all the elements arranged in a single vertical column. There are eighteen groups in total. Elements in the same group tend to have the same chemical properties because they contain the same number of electrons in their outermost shells (valence electrons).
Elements in the first group or group 1 have only one electron in their outermost shell. They are known as alkali metals. They are called alkali metals because they react with water to form bases or alkali. These metals must be stored under oil as they are very reactive and easily react with air and water.
Alkali metals are also very electropositive and form ions by giving out their one valence electron, carrying a charge of +1. The three alkali metals are Lithium (Li), Sodium (Na) and Potassium (K), with Potassium being the most electropositive.
Learn more about alkali metals here:
https://brainly.com/question/18153051
#SPJ4
The unit of radioactive exposure is given by a. grams b. rems c. ppm d. ppb '
Answers
The unit of radioactive exposure is given by (b) rems. The rem (Roentgen equivalent man) is a unit of measurement used to quantify the amount of radiation dose absorbed by living tissue. It takes into account the biological effects of different types of radiation on human health.
The unit of grams (a) is a measure of mass and is not specifically related to radiation exposure.
PPM (c) stands for parts per million, which is a unit used to express the concentration of a substance in a mixture. It does not directly measure radiation exposure.
Similarly, PPB (d) stands for parts per billion and is also a unit of concentration, not radiation exposure.
Therefore, the correct unit for measuring radioactive exposure is (b) rems.
To know more about radioactive exposure refer here :
https://brainly.com/question/31701460#
#SPJ11
Oxygen Supply in Submarines Nuclear submarines can stay under water nearly indefinitely because they can produce their own oxygen by the electrolysis of water.
How many liters of O2 at 298 K and 1.00 bar are produced in 2.25 hr in an electrolytic cell operating at a current of 0.0400 A?
Answers
Oxygen supply in submarines: Oxygen is a vital gas that helps to sustain life on earth. Submarines are known for their ability to stay underwater for extended periods.
A nuclear submarine can stay underwater almost indefinitely because they can create their own oxygen through the electrolysis of water.In submarines, the oxygen supply is provided through the electrolysis of water. Electrolysis is the process of using electricity to split water into hydrogen and oxygen gases.
This is done through a process known as the electrolytic cell. The process of electrolysis of water is a two-step process. The first step is the oxidation of water molecules at the anode, which releases oxygen gas. The second step is the reduction of water molecules at the cathode, which produces hydrogen gas.
The amount of oxygen produced in an electrolytic cell can be calculated using Faraday's law. Faraday's law states that the amount of a substance produced at an electrode is directly proportional to the amount of electricity that flows through the cell.
The amount of electricity that flows through a cell is determined by the current and the time. Therefore, the amount of oxygen produced can be calculated using the following equation:moles of O2 = (current x time) / (2 x F)where F is Faraday's constant, which is equal to 96,485.34 C/mol.
The volume of O2 produced can be calculated using the ideal gas law: PV = nRT where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature.Substituting the values given in the equation, we get:moles of O2 = (0.0400 A x 2.25 hr) / (2 x 96485.34 C/mol) = 0.0000527 moles
The volume of O2 produced can be calculated as follows:V = nRT/P = (0.0000527 mol x 8.314 J/K/mol x 298 K) / 1.00 bar = 1.23 LTherefore, 1.23 liters of O2 are produced in 2.25 hours in an electrolytic cell operating at a current of 0.0400 A.
To know more about Submarines here
https://brainly.com/question/32457172
#SPJ11
Which structure is the sun located
Answers
true or false : A precipitate is formed when two liquids are combined and a solid compound comes out
Answers
Answer:
true I think ?38363849472
Answer:
true
Explanation:
A 36.04 g sample of water is boiled. When the steam expands to fill the vessel how many atoms of hydrogen would be found in the sample?
Answers
Answer:
The number of hydrogen atoms in 36.04 g sample of steam is 2.408 × 10²⁴ atoms of hydrogen
Explanation:
The given parameters are;
The mass of the sample of water, H₂O = 36.04 g
From the principle of conservation of matter, the mass of the water = The mass of the steam, therefore;
The mass of the steam = 36.04 g
The molar mass of H₂O = 18.01528 g/mol
Therefore, the number of moles, n, of H₂O in the 36.04 g of steam is given as follows;
n = Mass/(Molar Mass) = (36.04 g)/(18.01528 g/mol) = 2.00052399963 ≈ 2 moles
The number of molecules of H₂O per mole of H₂O is geven by the Avogadro's number = 6.02 × 10²³ molecules
Therefore;
The number of H₂O in 2 moles of H₂O = 2 × 6.02 × 10²³ = 1.204 × 10²⁴ molecules
The number of hydrogen atoms per molecule of H₂O = 2 hydrogen atoms
Therefore;
The number of hydrogen atoms in the 1.204 × 10²⁴ molecules of H₂O = 2 × 1.204 × 10²⁴ molecules of H₂O
Which gives;
The number of hydrogen atoms in the 1.204 × 10²⁴ molecules of H₂O = 2.408 × 10²⁴ atoms of hydrogen
Therefore;
The number of hydrogen atoms in 36.04 g sample of steam = 2.408 × 10²⁴ atoms of hydrogen.
What is the total number of electrons in an atom of an element with an atomic number of 18 and a mass number
of 40?
a. 58
c. 22
b. 18
d. 40
Answers
Answer:
18
Explanation:
In neutral atom protons and electrons are equal in number as it atomic number or proton number is 18 so electrons are also 18
The total number of electrons in an atom of an element with an atomic number of 18 and a mass number of 40 is 18.
What are the difference between atomic number and atomic mass ?Atomic mass can be defined as the total number of neutrons and protons located in nucleus of an element while atomic number is the number of protons present in the nucleus of element.
Atomic mass can be defined as the average weight of an element where as the atomic number is the total number of protons present in the nucleus; Atomic mass have the symbol A while atomic number have the symbol the letter Z.
Different isotopes of an element are differentiated by atomic mass is while isotopes share the same atomic number, in neutral atom protons and electrons are equal to atomic number or proton number is 18 and electrons are also 18
Learn more about atomic number, here:
brainly.com/question/8834373
#SPJ6
DNA is inside the ________________________ of the cell.
A human has _______ pair of chromosomes making us have a total of ________ chromosomes in our cells.
Our DNA is _________________ _____________ from our parents. These are called __________________________ traits.
Answers
Answer: 1. Nucleus
2. 23
3. 46
our DNA is significantly different from our parents
last one is genetic.
Explanation: I hope it's right
helppppp!!!!
A double bond is shown conventionally by __lines joining
the atoms.
a 4
b 2
C5
d3
Answers
Answer:
b.2
Explanation:
the two are joining the atoms
a rock with a mass of 130.0 grams placed in 30.o mL of water. The water level rises to 75.0 mL. What is the density of the rock?
Answers
Answer: 2.88888889
Explanation:
1. Density = mass/volume.
2. Density = 130/45
3. 130/45 = 2.88888889
4. Density = 2.88888889
As deepwater drilling for crude oil continues deeper into the earth, why is drilling mud used in place of seawater? Select one: a. Drilling mud Is the result of the drilling process and is not used during the drilling. b. Drilling mud is more dense and will settle deeper down the tunnel. c. Drilling mud is easier to access than sea water at those depths. d. Drilling mud provides additional pressure to keep the tunnel form collapsing.
Answers
Answer:
It is either B or D, and I believe the answer is D.
Explanation:
Deepwater drilling relies on mud being pumped into the drill well for pressure purposes. Without the mud, gas and oil would just rise up uncontrollably because without counteracting the pressure from the sea and the rock, it would be spewing out. The mud helps the drill create the heavy column of downward force needed to maintain control of the output of gas and oil.
The answer choices that would best match the description would be B or D. Since the mud is there for provide pressure and downward force, I would go with choice D as my answer.
Considering only specific heat, ______would be the most ideal for use in cookware.
Answers
Considering only specific heat, Lead would be the most ideal for use in cookware.
The amount of heat needed to raise a substance's temperature by one degree Celsius in one gram is known as its specific heat. Typically, calories or joules per gram per degree Celsius are used as the units of specific heat.
Lead would be the material best suitable for use in cookware if only particular heat were taken into account.
Lead, however, is too hazardous and hefty in comparison to the other possibilities.
Aluminum or iron would create the best cookware, therefore these materials may be the best overall options.
To know more about specific heat, visit,
https://brainly.com/question/27991746
#SPJ4
Answer:
its lead
Explanation:
insane ik
Penny reads 11 pages in 1/3 hours what is the unit rate for pages per hours ?
Answers
Answer: 33/ because 1/3 of a 11 is 33.
Explanation:
All you had to do was multiply 11 with 3 thats it.
HURRY IM ON A TIMER
Which types of waves requires matter to carry energy?
A.electromagnetic waves only
B.mechanical waves only
C.electromagnetic and mechanical waves
D.longitudinal and electromagnetic waves
Answers
Answer:
B: Mechanical waves only.
Explanation:
'Mechanical waves require medium in order to transport their energy from one location to another.'
Hope this helps!
Answer:
Mechanical waves requires matter to carry enegry.
As mechanical waves are defined as the wave which requires any medium to transfer enegry from one place to another place.
so, the answer is Mechanical waves.
hope it helps..
i need help with this
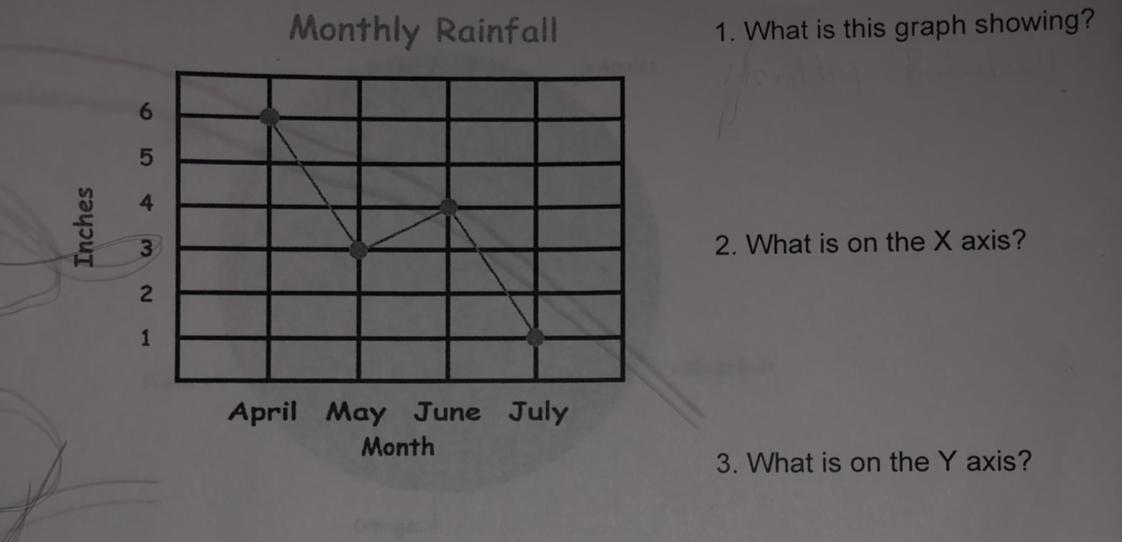
Answers
Answer:
1. The graph is showing the monthly rainfall between April to July.
2. The month is on the X axis.
3. The inches of the monthly rainfall is on the Y axis.
Which functional group does the molecule below have?
H H H H
H-Ċ-Ċ-Ċ-Ċ-0-H
| | | |
н ннн
O A. Amino
O B. Ester
O C. Ether
O D. Hydroxy
Answers
Because of the OH substituent