The molecule 2-butene is able to undergo a process called cis-trans isomerization, where the molecule switches from being a cis-alkene to a trans-alkene. This transformation can be induced by light. H H3C light XX C: ___H3C CH3 ____H CH3 What is the hybridization of the two central carbon atoms in 2-butene? O sp O sp2 O sp3 The isomerization requires breaking the a bond. Use the table of bond energies to determine the approximate amount of energy (in joules) required to break the C-C a bond in 2-butene, both per mole and per molecule.E= J/mol E = J/moleculeWhat is the wavelength of light that would be required to perform the cis-trans isomerization of one molecule of 2-butene? = nm nm Which region of the electromagnetic spectrum is this wavelength of light within? O visible O ultaviolet O X-ray O infrared
Answers
The molecule 2-butene is able to undergo a process called cis-trans isomerization, the molecule switches from being a cis-alkene to a trans-alkene. hybridization of the two central carbon atoms in 2-butene is sp².
The molecular formula of the 2 - butene is given as follows :
CH₃ - CH = CH - CH₃
The total number of carbon in the butene molecule is four. the first and the four carbon in the structure formed the covalent bond with three hydrogen and the carbon. in the theses position the hybridization is the sp³. the second and the third carbon that is the central carbon has the three sigma bond that is single bond and one is the double bond that is pi bond is the sp² hybridize.
To learn more about hybridization here
https://brainly.com/question/25648755
#SPJ4
Related Questions
The K of a given reactions is 432. Is the reaction favorable or not favorable?
Answers
Answer:Favorable
Explanation:um I know That it is Favorable sorry!
Is cooking an egg a chemical reaction
(I will give brainiest)
Answers
Answer:
cooking an egg is a chemical reaction because you can change the cooked egg into it original form
Explanation:
pls pls give brainliest pls
Answer:
Yes,Cooking an egg is a chemical reaction.
Explanation:
Cooking an egg is a chemical reaction because you can't change cooked egg into raw or its previous state.There are two types of reactions
Physical reactionChemical reactionA 1.00 liter solution contains 0.42 moles nitrous acid and 0.32 moles sodium nitrite .
If 0.16 moles of nitric acid are added to this system, indicate whether the following statements are true or false.
(Assume that the volume does not change upon the addition of nitric acid.)
A. The number of moles of HNO2 will decrease.
B. The number of moles of NO2- will remain the same.
C. The equilibrium concentration of H3O+ will increase.
D. The pH will decrease.
E. The ratio of [HNO2] / [NO2-] will increase
Answers
Answer:
E. The ratio of [HNO2] / [NO2-] will increase
D. The pH will decrease.
Explanation:
Nitrous acid ( HNO₂ ) is a weak acid and NaNO₂ is its salt . The mixture makes a buffer solution .
pH = pka + log [ salt] / [ Acid ]
= 3.4 + log .32 / .42
= 3.4 - .118
= 3.282 .
Now .16 moles of nitric acid is added which will react with salt to form acid
HNO₃ + NaNO₂ = HNO₂ + NaNO₃
concentration of nitrous acid will be increased and concentration of sodium nitrite ( salt will decrease )
concentration of nitrous acid = .42 + .16 = .58 M
concentration of salt = .32 - .16 = .16 M
ratio of [HNO₂ ] / NO₂⁻]
= .42 / .32 = 1.3125
ratio of [HNO₂ ] / NO₂⁻] after reaction
= .42 + .16 / .32 - .16
= 58 / 16
= 3.625 .
ratio will increase.
Option E is the answer .
pH after reaction
= 3.4 + log .16 / .58
= 2.84
pH will decrease.
What mass of NaCl is needed to produce a 26.4 mol/L with a 1.7 L volume?
Answers
we would need 2625.13 grams (or 2.62513 kilograms) of NaCl.
To calculate the mass of NaCl required to produce a 26.4 mol/L solution with a 1.7 L volume, we need to use the formula that relates the mass of solute, moles of solute, and molarity:Molarity (M) = moles of solute / liters of solution Rearranging this formula, we get:moles of solute = Molarity (M) x liters of solutionWe can use this formula to find the moles of NaCl needed:moles of NaCl = 26.4 mol/L x 1.7 L = 44.88 molNow, we can use the molar mass of NaCl to convert from moles to grams. The molar mass of NaCl is 58.44 g/mol:mass of NaCl = moles of NaCl x molar mass of NaClmass of NaCl = 44.88 mol x 58.44 g/mol = 2625.13 gTo produce a 26.4 mol/L solution with a 1.7 L volume.
for more question on NaCl
https://brainly.com/question/23269908
#SPJ8
What is the answer to this ?

Answers
D
Explanation:
Because the number of protons is the same as the atomic number, we know what the number of protons has to be 26. The mass number, 55, is the same as the number of protons plus neutrons. Lastly, because there is no charge on this atom, the number of electrons would have to be the same as the number of protons, which is 26.
For the reaction C + 2H2 - CH4
how many grams of carbon are required to produce 10.7 moles of methane, CH4?
Use the following molar masses:
hydrogen: 1
carbon: 12
Answers
Taking into account the reaction stoichiometry, 128.4 grams of C are required to produce 10.7 moles of methane.
Reaction stoichiometryIn first place, the balanced reaction is:
C + 2 H₂ → CH₄
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
C: 1 moleH₂: 2 molesCH₄: 1 moleThe molar mass of the compounds is:
C: 12 g/moleH₂: 2 g/moleCH₄: 16 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
C: 1 mole ×12 g/mole= 12 gramsH₂: 2 moles ×2 g/mole= 4 gramsCH₄: 1 mole ×16 g/mole= 16 gramsMass of C requiredThe following rule of three can be applied: If by reaction stoichiometry 1 mole of CH₄ is produced by 12 grams of C, 10.7 moles of CH₄ are produced by how much mass of C?
mass of C= (10.7 moles of CH₄×12 grams of C)÷1 mole of CH₄
mass of C= 128.4 grams
Finally, 128.4 grams of C are required.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
How strong are bonds between subatomic particles?
Answers
Answer:
they are indeed very strong
List two characteristics of a calorimeter that are necessary for successful heat measurement.
Answers
Two characteristics of a calorimeter that are necessary for successful heat measurement are good thermal insulation to minimize heat loss and a temperature measurement device to accurately measure temperature changes.
How does a calorimeter work?A calorimeter works by measuring the heat exchanged between two substances in a chemical or physical process. The substances are placed in the calorimeter and allowed to reach thermal equilibrium, and the temperature change of the system is measured to calculate the amount of heat transferred.
What is the difference between a bomb calorimeter and a coffee cup calorimeter?A bomb calorimeter is designed for measuring the heat of combustion of a substance in a sealed container under constant volume conditions, while a coffee cup calorimeter is a simpler design used for measuring the heat of reaction of a substance in an open container under constant pressure conditions. The bomb calorimeter is typically more accurate but also more complex and expensive to use.
Learn more about calorimeter here:
https://brainly.com/question/4802333
#SPJ1
which orbital diagram represents neon (atomic number =10)?
Answers
Answer:
Neon is the tenth element with a total of 10 electrons. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. The remaining six electrons will go in the 2p orbital.
Explanation:
During a reaction with solids generally the _______ the size of each piece, the larger the total surface area. This means _______ collisions and a greater chance of reaction.
A. smaller, more
B. larger, more
C. larger, less
D. smaller, less
Answers
Answer:
A
Explanation:
I'm assuming this question implies that the surface area is in relation to the volume of the pieces. In that case, the SMALLER the size of each piece, the larger the surface area. This is because more particles are able to fit into the container if they are smaller, leading to more surface area. Since more pieces can fit into the container, MORE collisions happen according to collision theory. I cannot add a link, but for a helpful analogy, look up "How To Speed Up Chemical Reactions (and get a date) - Aaron Sams.
Answer: A. smaller, more
Explanation: Founders Educere answer
PLEASE ANSWER QUICKLY!!!!
2KI (aq) + Cl₂(g) → 2KCl(aq) + 1₂(g)
What volume of 12 gas forms when
21 L Cl2 react at STP?
[?] L 12

Answers
The volume of 12 gas forms when 21 L Cl2 react at STP is 21 L.
To determine the volume of 12 gas (I assume you mean I2 gas) formed when 21 L of Cl2 reacts at STP (standard temperature and pressure), we need to use the ideal gas law equation.
The ideal gas law equation is given by:
PV = nRT
Where:
P = pressure
V = volume
n = number of moles
R = ideal gas constant
T = temperature
At STP, the pressure is 1 atm, and the temperature is 273.15 K.
From the balanced equation, we can see that the molar ratio between Cl2 and I2 is 1:1. So, if 21 L of Cl2 reacts, it will produce an equal volume of I2 gas.
Given that the volume of Cl2 is 21 L, we can assume the volume of I2 gas formed will also be 21 L.
Therefore, the volume of I2 gas formed is 21 L.
For more such questions on volume
https://brainly.com/question/1749900
#SPJ8
Balance the following half eqn. in alkaline medium. Mno-4___ Mno2
Answers
MnO4- + 4e- → MnO2 + 2H2O Now the half-equation is balanced in alkaline medium.
To balance the half-equation MnO4- → MnO2 in alkaline medium, we need to follow the steps for balancing redox reactions in basic solution. The goal is to balance the number of atoms and charges on both sides of the equation.
Start by balancing the atoms other than oxygen and hydrogen. In this case, we only have manganese (Mn) atoms. There is one Mn atom on both sides, so the Mn atoms are already balanced.
Balance the oxygen atoms by adding water (H2O) molecules to the side that lacks oxygen. Since there are four oxygen atoms on the left side (MnO4-) and only two on the right side (MnO2), we need to add two water molecules to the right side:
MnO4- → MnO2 + 2H2O
Next, balance the hydrogen atoms by adding hydrogen ions (H+) to the side that lacks hydrogen. In this case, the left side (MnO4-) already has sufficient hydrogen atoms, so no hydrogen ions need to be added.
Balance the charges by adding electrons (e-) to the side that has a higher charge. MnO4- has a charge of -1, while MnO2 has no charge. Since the left side has a higher charge, we need to add electrons to the right side:
MnO4- + 4e- → MnO2 + 2H2O
Now the half-equation is balanced in alkaline medium. The Mn atoms, oxygen atoms, hydrogen atoms, and charges are all balanced. The addition of water and hydrogen ions helps balance the oxygen and hydrogen atoms, while the addition of electrons balances the charges.
For more such questions on alkaline medium. visit:
https://brainly.com/question/27960992
#SPJ8
Scoring Scheme: 3-3-2-1 Part II. You considered the properties of two acid-base indicators, phenolphthalein and methyl orange. Many indicators are weak acids in water and establish the equilibrium: HIn(aq)(Color 1) H2O(l) H3O (aq) In-(aq)(Color 2). Indicators change color depending on whether they are in a protonated (HIn) or unprotonated (In-) form. What is the equilibrium expression for the phenolphthalein indicator in water and what colors are the protonated and unprotonated forms of the indicator
Answers
Answer:
Explanation:
Phenolphthalein is a protonated indicator and methyl orange is a basic indicator having hydroxyl ionisable part .
Phenolphthalein can be represented by the following formula
HPh which ionizes in water as follows
HPh + H₂O ⇄ H₃O⁺ + Ph⁻
( colourless ) ( pink )
In acidic solution it is in the form of protonated Hph form which is colourless
In basic medium , it ionises to give H₃O⁺ and unprotonated Ph⁻ whose colour is pink .
100mL of a solution that is simultaneously 15.6 mg/mL malonic acid, 3.38 mg/mL MnSO4 • H2O, and 0.03% starch
Calculate the g and mL necessary to make this solution
Answers
52.6g and 23.8mL necessary 100mL of a solution that is simultaneously 15.6 mg/mL malonic acid, 3.38 mg/mL MnSO4 • H2O, and 0.03% starch to make this solution.
What is malonic acid?
The chemical formula of malonic acid is CH2(COOH)2. Malonates include the ionized form of malonic acid as well as its esters and salts. Because it interferes with respiration, malonic acid is extremely harmful, especially in cases of cancer and other degenerative disorders (the making of ATP in mitochondria). Malonic acid is a somewhat unstable substance with limited practical uses. Beetroot contains its calcium salt, however the acid itself is often made by hydrolyzing diethyl malonate.
To learn more about malonic acid, refer: -
https://brainly.in/question/46046349
SPJ1
Two asteroids are 75,000 m apart one has a mass of 8 x 10^7 N what is the mass of the other asteroid
Answers
The mass of the asteroid is C. 1.2 x \(10^{12}\) Kg
To find the mass of the other asteroid, we can rearrange the equation for the gravitational force between two objects:
F = (G * m1 * m2) / \(r^{2}\)
where F is the force of gravity, G is the gravitational constant, m1 and m2 are the masses of the two asteroids, and r is the distance between them.
Given that the distance between the asteroids is 75000 m, the force of gravity between them is 1.14 N, and one asteroid has a mass of 8 x \(10^{7}\) kg, we can substitute these values into the equation and solve for the mass of the other asteroid (m2):
1.14 N = (6.67430 × \(10^{-11}\) N \(m^{2}\)/\(Kg^{2}\) * 8 x \(10^{7}\) kg * \(m2\)) / \((75000 m)^{2}\)
Simplifying and solving the equation, we find that the mass of the other asteroid (m2) is approximately 1.2 x \(10^{12}\) kg. Therefore, Option C is correct.
The question was incomplete. find the full content below:
Two asteroids are 75000 m apart one has a mass of 8 x \(10^{7}\) kg if the force of gravity between them is 1.14 what is the mass of the asteroid
A. 3.4 x \(10^{11}\) kg
B. 8.3 x \(10^{12}\) kg
C. 1.2 x \(10^{12}\) kg
D. 1.2 x \(10^{10}\) kg
Know more about gravitational force here:
https://brainly.com/question/72250
#SPJ8
Please help me with this question.

Answers
Answer:1=B
2=A
Explanation:
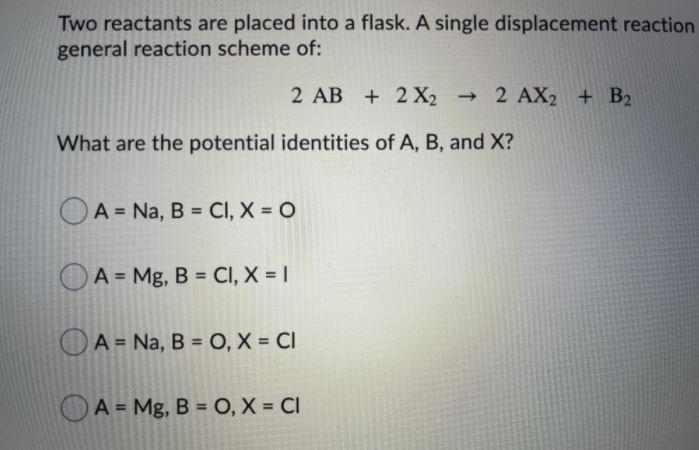
two reactants are placed into a flask a single displacement reaction occurs, with general reaction of scheme of:
Answers
Given that a single-displacement reaction occurred between two (2) reactants which were placed into a flask, the potential identities of A, B, and X are: D. A = Mg, B = O, X = Cl.
A single-displacement reaction is also referred to as a single-replacement reaction and it can be defined as a type of chemical reaction wherein a single (one) chemical element of the reacting chemical compound is displaced (replaced) by a similar chemical element.
This ultimately implies that, a single-displacement reaction typically involves a single element displacing another chemical element within a chemical compound.
Note: A potential identity reveals the chemical symbol or name of a chemical element in a particular chemical reaction.
Given the following general reaction of scheme of:
\(2AB+2X_2\rightarrow 2AX_2+B_2\)
We would proceed to replace the variables with chemical elements as follows:
\(2MgO+2Cl_2\rightarrow 2MgCl_2+O_2\)
Therefore, the potential identities of A, B, and X are:
\(A = Mg\\\\B = O\\\\X = Cl\)
Read more: https://brainly.com/question/25670663
PS: Your question is lacking the necessary information, but I have provided them in the image attached.
If 4.53g Ar are added to 1.12 atm He in a 2.00 L cylinder at 27.0 Celsius degree
What is the total of gaseous mixture?
Answers
The total gaseous mixture is 4.48 g.
The total gaseous mixture can be calculated using the ideal gas law: PV = nRT.
First, we need to convert the temperature to Kelvin: 27.0°C + 273.15 = 300.15 K.
Next, we can use the ideal gas law to calculate the number of moles of each gas:
For He: n = (1.12 atm) (2.00 L) / (0.08206 L·atm/mol·K) (300.15 K) = 0.0906 mol
For Ar: n = (4.53 g) / (39.95 g/mol) = 0.113 mol
The total number of moles of gas in the mixture is then:
n(total) = n(He) + n(Ar) = 0.0906 mol + 0.113 mol = 0.204 mol
Finally, we can calculate the total mass of the mixture:
m(total) = n(total) × M(avg)
where M(avg) is the average molar mass of the mixture, which can be calculated as:
M(avg) = (M(He) + M(Ar)) / 2 = (4.003 g/mol + 39.95 g/mol) / 2 = 21.98 g/mol
Thus,
m(total) = 0.204 mol × 21.98 g/mol = 4.48 g
Therefore, The total weight of the gaseous mixture is 4.48 g.
To know more about the Gaseous mixture, here
https://brainly.com/question/30966571
#SPJ1
The total energy of the car is its potential energy + its kinetic energy. This total energy remains constant across the length of the track. What would be the best way to increase the energy of the coaster as it proceeds through the course?
Responses
A Increase the height at point B. Increase the height at point B.
B Make the roller coaster longer. Make the roller coaster longer.
C Decrease the number of cars in each train. Decrease the number of cars in each train.
D Increase the height at point A.
Answers
The energy of the coaster can be increased through the course if we increase the height at point A.
What is the roller coaster?The roller coaster is a device that can be used to describe the conversion of energy from potential energy to kinetic energy. Let us note that the total energy of the system must always be constant and it is the sum of the kinetic energy and the potential energy.
Now we want to know how to increase the energy of the coaster as it proceeds through the course. This would have to do with an increase in the height of the point A.
Learn more about roller coaster:https://brainly.com/question/19920727
#SPJ1
25g of NH3 is mixed with 4 mole of O2 is the given reaction
a.which is the limiting reaction
b.what mass of no is formed
c.what mass of h2o is formed
Answers
B) 44.1 g
C) 39.6 g
In which step of meiosis do chromosomes first condense?
telophase I
telophase II
prophase I
prophase II
Answers
Answer:
Someone Said Telophase II. Ill update if its right or wrong when I finish.
Update : He is.... Wong. False.
Explanation:
The answer is Prophase I. It is not Telophase II.
How many grams are in 0.0823 moles of Ar? given; unknown:
Answers
Answer:
3.2877204
Explanation:
Acetylide ions react with aldehydes and ketones to give alcohol addition products.
a. True
b. False
Answers
Answer:
a
Explanation:
Post-Lab Questions
1. A beverage company is having trouble with the production of the dye in their drinks. The color of their drink mix is supposed to be a pale green color, but they often get different results. For each unwanted result, choose the most plausible explanation to help the company improve the formula.
(1pts)
The color of the drink is too pale after adding the dye to the drink because
Choose...too much dye was added to the drink.the water in the drink is evaporating.not enough dye was added to the drink.the wrong dye was added to the drink.
(1pts)
The color of the dye is appearing as red, instead of green because
Choose...too much dye was added to the drink.the water in the drink is evaporating.not enough dye was added to the drink.the wrong dye was added to the drink.
(1pts)
The drink started out the correct color but it is getting darker over time, even though nothing has been added to the drink, because
Choose...too much dye was added to the drink.the water in the drink is evaporating.not enough dye was added to the drink.the wrong dye was added to the drink.
(1pts)
2. Beer's Law states that A=εbc, where A is the absorbance, ε is the molar absorptivity of the solute, b is the path length, and c is the concentration. Identify the experimental evidence from the activity that you have for the dependence of absorbance on each variable.
The evidence for the dependence of absorbance on the variable ε is
increasing the cuvette width increases the absorbance.
changing the compound changes the absorbance behavior.
adding more water decreases the absorbance.
Choose...ABC
(1pts)
The evidence for the dependence of absorbance on the variable b is
increasing the cuvette width increases the absorbance.
changing the compound changes the absorbance behavior.
adding more water decreases the absorbance.
Choose...ABC
(1pts)
The evidence for the dependence of absorbance on the variable c is
increasing the cuvette width increases the absorbance.
changing the compound changes the absorbance behavior.
adding more water decreases the absorbance.
Choose...ABC
(1pts)
3. Describe how you could use the Beer's Law simulation to experimentally determine the best wavelength at which to perform an experiment.
Measure the absorbance for solutions of multiple different solutes and find the minimum absorbance.
Measure the absorbance for solutions with different concentrations and find the slope of the trendline.
Measure the absorbance for the same solution at different wavelengths and find the maximum absorbance.
Measure the absorbance for the same solution in different cuvette sizes and find the y-intercept.
Answers
Answer:
1. not enough dye was added to the drink.
The wrong dye was added to the drink
the water in the drink is evaporating
2. Changing the compound changes the absorbance behavior.
3. Measure the absorbance for the same solution in different cuvette sizes and find the y-intercept.
Explanation:
When the beverage company adds dye to the drink, there should be standard quantity added to the drink so that the color of the drink remains constant. When too much dye is added to the drink, the color will get dark brown or black. When the color of drink get lighter than green this means dye is not added in required quantity.
8) At 15 °C, a certain reaction is able to produce 0.80 moles of product per minute? At what rate might
the product be produced at 5 °C?
a. 1.6 moles per minute
b. 0.80 moles per minute
c. 0.40 moles per minute
d. 1.20 moles per minute
Answers
At 15 °C, a certain reaction is able to produce 0.80 moles of product per minute.At 0.40 moles per minute the product be produced at 5 °C.
What is moles ?Moles are small burrowing mammals found in many parts of the world. They are typically brown or black in color and can be identified by their distinctive hairy snouts and short tails. Moles have a unique way of moving through soil and other material. They use their long claws to dig tunnels that serve as their home and pathways for foraging for food. Moles feed on a variety of insects and plant material, such as earthworms, grubs, and roots. They also help to aerate soil and improve water drainage. Moles are solitary animals and are rarely seen.
To learn more about moles
https://brainly.com/question/29367909
#SPJ1
41.6 g Al(NO3)3m Are added to a flask, how many liters of water should be added to create a 0.450 M solution?
Answers
To calculate the volume of water needed to create a 0.450 M solution of Al(NO3)3, we need to use the formula:
Molarity = moles of solute / volume of solution in liters
First, we need to determine the number of moles of Al(NO3)3 we have:
moles of Al(NO3)3 = mass / molar mass
molar mass of Al(NO3)3 = 1 x atomic mass of Al + 3 x atomic mass of N + 9 x atomic mass of O = 1 x 26.98 + 3 x 14.01 + 9 x 16.00 = 212.99 g/mol
moles of Al(NO3)3 = 41.6 g / 212.99 g/mol = 0.195 mol
Next, we can rearrange the formula above to solve for the volume of solution:
volume of solution = moles of solute / molarity
volume of solution = 0.195 mol / 0.450 M = 0.433 L
Therefore, we need to add 0.433 L (or 433 mL) of water to 41.6 g of Al(NO3)3 to create a 0.450 M solution.
Calculate the ratio of the moles of produced to the moles of each of the reactants used. (Write two separate ratios.)
Answers
Ratio of moles of NH₃ produced to moles of N₂ used: 2 moles of NH₃ / 1 mole of N₂
Ratio of moles of NH₃ produced to moles of H₂ used: 2 moles of NH₃ / 3 moles of H₂
What is the mole ratio of the reaction?From the balanced chemical equation:
N₂ + 3 H₂ ⟶ 2 NH₃
We can determine the ratio of moles of products to the moles of each reactant.
Ratio of moles of NH₃ produced to moles of N₂ used:
From the balanced equation, we can see that 1 mole of N₂ reacts to produce 2 moles of NH₃. Therefore, the ratio is:
2 moles of NH₃ / 1 mole of N₂
Ratio of moles of NH₃ produced to moles of H₂ used:
From the balanced equation, we can see that 3 moles of H₂ react to produce 2 moles of NH₃. Therefore, the ratio is:
2 moles of NH₃ / 3 moles of H₂
Learn more about the mole ratio at https://brainly.com/question/19099163
#SPJ1
Given the equation of reaction;
N₂ + 3 H₂ ---> 2 NH₃
Calculate the ratio of the moles of produced to the moles of each of the reactants used. (Write two separate ratios.)
You have a carbonate buffer with pH 10.3 and a concentration of 2.0 M. What is the buffer capacity of 100 mL of the buffer against 3.0 M CsOH?
Answers
Answer:
Explanation:
pH = 10.3
[ H] = 10⁻¹⁰°³
= 5 x 10⁻¹¹ M
concentration of CsOH C = 3 M
pKa of carbonate = 6.35
Ka = 10⁻⁶°³⁵ = 4.46 x 10⁻⁷
Buffer capacity = 2.303 x C x Ka x [ H⁺] / ( Ka + [ H⁺]² )²
= 2.303 x 3 x 4.46 x 10⁻⁷ x 5 x 10⁻¹¹ / ( 4.46 x 10⁻⁷ + 25 x 10⁻²² )²
= 154 x 10⁻¹⁸ / 19.9 x 10⁻¹⁴
= 7.74 x 10⁻⁴ .
What unit of time is based on the revolution of Earth around the sun?
A. month
B. year
C. day
D. hour
Answers
2.
Which mixture could be a useful buffer in a solution?
acetic acid (CH3CO2H) and hydrochloric acid (HCl)
sodium hydroxide (NaOH) and elemental sodium (Na)
ammonia (NH3) and ammonium chloride (NH4Cl)
acetic acid (CH3CO2H) and ammonia (NH3)
Pls answer quickly
Answers
Ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)) mixture could be a useful buffer in a solution. Option C
A buffer is a solution that can resist changes in pH when small amounts of acid or base are added. It consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The buffer system works by the principle of Le Chatelier's principle, where the equilibrium is shifted to counteract the changes caused by the addition of an acid or a base.
In option A, acetic acid (\(CH_3CO_2H\)) is a weak acid, but hydrochloric acid (HCl) is a strong acid. This combination does not form a buffer because HCl is completely dissociated in water and cannot provide a significant concentration of its conjugate base.
Option B consists of sodium hydroxide (NaOH), which is a strong base, and elemental sodium (Na), which is a metal. This combination does not form a buffer as there is no weak acid-base pair involved.
Option D contains acetic acid (\(CH_3CO_2H\)), a weak acid, and ammonia (\(NH_3\)), a weak base. Although they are weak acid and base, they do not form a buffer system together as they are both weak acids or bases and lack the required conjugate acid-base pair.
Option C, ammonia (\(NH_3\)), is a weak base, and ammonium chloride (\(NH_4Cl\)) is its conjugate acid. This combination can form a buffer system. When ammonia reacts with water, it forms ammonium ions (NH4+) and hydroxide ions (OH-).
The ammonium ions act as the weak acid, while the ammonia acts as the weak base. The addition of a small amount of acid will be counteracted by the ammonium ions, and the addition of a small amount of base will be counteracted by the ammonia, thus maintaining the pH of the solution relatively stable.
Therefore, option C, consisting of ammonia (\(NH_3\)) and ammonium chloride (\(NH_4Cl\)), is the suitable mixture that could be a useful buffer in a solution.
For more such question on buffer visit:
https://brainly.com/question/13076037
#SPJ8