The molecular formula for both cis-2-butene and trans-2-butene is c4h8. compounds which have the same molecular formula but have different structures are called:______.
Answers
The molecular formula for both the cis-2-butene and trans-2-butene is c4h8. The compounds which have the same molecular formula but have different structures are called isomers.
What are isomers?Isomerism, the existence of molecules that have same numbers of the same kinds of atoms (and hence the same formula) but differ in chemical and physical properties.
Stated colloquially, isomers are chemical compounds that have same parts but are nonetheless not the same. To make crude analogy, two bracelets, each consisting of five red and five green beads, could be arranged in many different isomeric forms, depending on the order of the colors.
Each bracelet would have the same parts—that is, five red and five green beads—but each variation would be different. One could also imagine combinations of those same beads in which the pendant chains were attached to a bracelet in a variety of ways. One might imagine two bracelets of the same red-green order but with the identical chains attached in different orientations. Such structures also would be analogous to the isomers. In a more subtle analogy, one’s hands can be seen as an isomeric. Each hand possesses same kinds of fingers, but a right hand can never be superimposed perfectly on a left hand; they are different.
To know more about isomers visit: https://brainly.com/question/12796779
#SPJ4
Related Questions
what is the heat of solution (qsoln) for the neutralization reaction between nh3 and hcl?
Answers
The heat of solution (qsoln) for the neutralization reaction between NH3 and HCl depends on the specific conditions of the reaction, such as the concentrations of NH3 and HCl, the temperature, and the pressure.
In general, the neutralization of NH3 and HCl is an exothermic reaction, meaning it releases heat, and the value of qsoln is negative. The exact value of qsoln can be calculated using the heat of formation of NH3 and HCl and the enthalpy change of the reaction. However, without specific information about the reaction conditions, an accurate value for qsoln cannot be provided.
To find the heat of solution (qsoln) for the neutralization reaction between NH3 and HCl, follow these steps:
Step 1: Write the balanced chemical equation for the reaction:
NH3(aq) + HCl(aq) → NH4Cl(aq)
Step 2: Determine the molar enthalpy of the reaction (ΔH):
You can look up the molar enthalpy of the reaction in a reference table or calculate it using bond dissociation energies. For this reaction, the molar enthalpy is typically reported as -51.6 kJ/mol.
Step 3: Calculate the heat of solution (qsoln):
qsoln = n * ΔH
Where n represents the number of moles of NH3 and HCl reacting, and ΔH is the molar enthalpy of the reaction. To calculate n, you will need to know the volume and concentration of the NH3 and HCl solutions used in the reaction.
For example, if you have 0.1 moles of NH3 reacting with 0.1 moles of HCl, the calculation would be:
qsoln = 0.1 * (-51.6 kJ/mol) = -5.16 kJ
In this example, the heat of solution for the neutralization reaction between NH3 and HCl is -5.16 kJ.
Learn more about qsoln at: brainly.com/question/26708091
#SPJ11
3. What must you have when you pray?
Answers
Answer:
when you pray you will feel a peace and happiness in your heart
3. How are natural selection and selective breeding related?
can someone help?
Answers
Answer:
In natural selection, organisms adapt to the environment to best suit nature's needs to survive. In selective breeding, it's as if nature were humans and the humans can choose which organisms can survive and die. They do it so they can develop organisms with desirable characteristics.
What does the oxidation number for elements of first transition series range between ?
Answers
The range of the of the oxidation number of the first transition series is +2 to +6.
What is transition metal?
Transition elements or transition metals are elements or metals that have partially filled d orbitals.
Examples of first transition metalsThe first main transition series begins with either;
scandium (Sc, atomic number 21)titanium (Ti, atomic number 22) chromium (Cr, atomic number 24) and ends with zinc (Zn, atomic number 30)Range of oxidation number of transition metalsscandium - oxidation number = +3titanium - oxidation number = +2, +3, and +4Chromium - oxidation number = + 6zinc - oxidation number = +2Thus, the range of the of the oxidation number of the first transition series is +2 to +6.
Learn more about oxidation number here: https://brainly.com/question/27239694
#SPJ1
What are bubbles (fizzes) in a soda can be described as??
Liquid to Gas or Gas to Liquid
Answers
Answer:
Liquid to Gas
Explanation:
The drink in the soda is a liquid and when you open the can, the CO2 leaves the container.
Hope this helped! :)
have a good day!
palladium crystallizes in a face-centered cubic unit cell. its density is 12.0 g / cm3 at 27oc. calculate the atomic radius of pd.
Answers
Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.0 g/cm3 at 27°C. Calculate the atomic radius of Pd.
A face-centered cubic (FCC) lattice is used by Palladium. As a result, the lattice parameter of palladium is a
=(4V/√3)^(1/3) ,
where V is the atomic volume of palladium. The formula for the density of a substance is d=m/V, where d is the density, m is the mass, and V is the volume of the substance. In this situation, m = M (mass of 1 mole of palladium), which can be expressed as M= n × m, where n is the number of moles of palladium and m is the mass of one palladium atom. Therefore, the density formula becomes
d=M/V.
Palladium's atomic volume is V=(4πr^3/3) /N_a,
where Na is Avogadro's constant (6.022 × 10^23 mol^-1). The atomic radius of Pd is given by the following formula:r=(a/2) × √2The density of Pd is given by the following formula
d=M/V
The molar mass of Pd can be calculated from its atomic weight (106.42 g/mol), M=106.42 g/mol The atomic volume of Pd is given by the following formula:
V= 4r^3/3Na
Use this value of V to determine the lattice parameter a = (4V/√3)^(1/3).r = (a/2) × √2
Calculations:d = 12.0 g/cm3M = 106.42 g/mol
V = (4πr^3/3) /N_a
Let's solve for V:
V = (4πr^3/3) /N_a = (4π (162.5 × 10^-30 m)^3/3) / (6.022 × 10^23 mol^-1) = 8.927 × 10^-6 cm^3/mol
The lattice parameter can be determined now
:a = (4V/√3)^(1/3) = (4 (8.927 × 10^-6 cm^3/mol) / √3)^(1/3) = 3.891 × 10^-8 cmThe atomic radius can be determined:r = (a/2) × √2 = (3.891 × 10^-8 cm/2) × √2 = 1.096 × 10^-8 cm
The atomic radius of Pd is 1.096 × 10^-8 cm.
Learn more about face-centered cubic (FCC) lattice at brainly.com/question/14885097
#SPJ11
which of these is used to determine the age of an object? question 8 options: palynology taphonomy radiocarbon paleontology
Answers
Radiocarbon dating is used to determine the age of an object.
Radiocarbon dating is a method used to estimate the age of organic materials based on the decay of radioactive carbon-14 isotopes. This technique is widely employed in archaeology, geology, and other scientific fields. When living organisms, such as plants or animals, are alive, they maintain a ratio of carbon-14 to stable carbon-12 isotopes.
However, once they die, the carbon-14 begins to decay at a known rate. By measuring the remaining carbon-14 and comparing it to the initial ratio, scientists can calculate the time that has passed since the organism's death. This method is particularly useful for dating objects that are up to around 50,000 years old. Palynology is the study of pollen grains, taphonomy focuses on the process of decay and fossilization, and paleontology deals with the study of fossils but not specifically dating methods.
To learn more about Radiocarbon dating click here
brainly.com/question/12693872
#SPJ11
Write an ionic equation for the reaction of zinc and dilute hydrochloric acid, include state symbols.
Answers
Explanation:
When zinc reacts with dilute hydrochloric acid, it is a metal-acid reaction to produce zinc chloride and hydrogen gas.
Zn (s) + 2HCl (aq) => ZnCl2 (aq) + H2 (g)
Ionic equation:
Zn2+ (aq) + 2Cl- (aq) => ZnCl2 (aq)
container a holds 712 ml 712 ml of ideal gas at 2.10 bar2.10 bar . container b holds 134 ml 134 ml of ideal gas at 4.80 bar4.80 bar . if the gases are allowed to mix together, what is the partial pressure of each gas in the total volume?
Answers
The saturation pressure of every gas in the entire volume, if somehow the gases being allowed to mix, is 2.5 atm.
Describe gases.In comparison to other forms of matter, like solids and liquids, vapors have a lower density. Particles have a great deal of kinetic energy yet aren't very attracted to one another, thus there is a lot of unoccupied space between them. A sampling of matter that assumes the shape of the bottle it is stored in and develops a consistent density on the inside of the container is referred to as a gas.
Pressure = 2.10 atm
Temperature = T K
Volume = 712 mL
= 0.712 L (1 mL = 0.001 L)
PV=nRT,
2.10 atm × 0.712 L = n × 0.0821 L.atm/K.mol × T K
n = 19.2232 / T moles
For Container B :
Pressure = 4.80 atm
Temperature = T
Volume = 134 mL
= 0.134 L (1 mL = 0.001 L)
= 4.80 atm × 0.134 L = n × 0.0821 L.atm/K.mol × 298 K
= n = 6.3435 / T moles
Total moles = 19.2132 / T moles + 6.3435 / T moles
= 25.5567 /T moles
Total Volume = 712 + 134 mL = 846 mL = 0.846 L
P × 0.846 L = 25.5567 /T × 0.0821 L.atm/K.mol × T
Total P = 2.5 atm
To know more about gases visit:
https://brainly.com/question/18124975
#SPJ4
What structure in a bacterial cell controls which substances enter and leave the cell?
Answers
Answer:
A cell membrane. A cell structure that controls which substances can enter or leave the cell. (found in all cells) In cells with cell walls, the cell membrane is located just inside the cell wall. In other cells, the cell membrane forms the outside boundary that separates the cell from its environment.
Hope this helps!
Do bacteria help people?? This is science but there is no tab
Answers
Answer:
Yes
Explanation:
Some bacteria is good for you like the bacteria in your digestive system, which helps with breaking down food and keeping you healthy
Using the dimensional Analysis
In the Above questions to convert 14,612 mL to DAL which of the following portrays to correct set up

Answers
Option A displays the accurate dimensional analysis for converting 14,612 mL to daL.
Determining the value of measurement across many units is crucial in many situations.This means that we must convert from one unit to another. The application of a conversion factor enables such a conversion.Now that we are aware of a conversion factor, we can utilize it to change mL into daL. In order for the units to cancel out, this conversion factor needs to be dimensionally precise in its arrangement.Option A displays the accurate dimensional analysis for converting 14,612 mL to daL.Learn more about unit conversion at:
brainly.com/question/11543684
#SPJ9
HELP!!!
Which of the following compounds are isomeric?

Answers
how many moles of silver nitrate are needed to produce 6.75 moles of copper (II) nitrate upon reacting with excess copper?
Answers
Answer:
13.50 moles of AgNO₃ are needed to produce 6.75 moles of Cu(NO₃)₂
Explanation:
We state the reactants:
AgNO₃
Cu
Then, the products are:
Cu(NO₃)₂
Ag
The reaction is: 2AgNO₃ + Cu → 2Ag + Cu(NO₃)₂
Ratio is 1:2.
The copper is in excess, so the limiting reactant is the silver nitrate:
1 mol of Cu(NO₃)₂ is produced by 2 moles of AgNO₃
Then, 6.75 moles of copper (II) nitrate would be produced by, the double of moles
(6 75 . 2) /1 = 13.50 moles
How many meters are in 125 cm?
Answers
Answer:
1.25 m
Explanation:
there are 100 cm in a metre. So to convert cm to m you divide the cm by 100
there are 1.25 meters in 125 cm
a fifth of distilled spirits is equal to about ___ ml.
Answers
It's always important to drink responsibly and avoid drinking and driving. A fifth of distilled spirits is equal to about 750 ml.
Distilled spirits are beverages that have been distilled to increase their alcohol content.
Ethanol, a by product of sugar fermentation, is the primary component of alcoholic beverages.
Distilled spirits are also known as hard liquor or spirits in the beverage industry and include gin, vodka, brandy, tequila, and whiskey.
There are a few facts about distilled spirits:
All distilled spirits are distilled, but not all distilled beverages are distilled spirits.
Distilled spirits include a variety of drinks, including whiskey, brandy, vodka, and gin, among others.
It's always important to drink responsibly and avoid drinking and driving.
Conclusively, a fifth of distilled spirits is equal to about 750 ml.
To know more about distilled spirits, visit:
https://brainly.com/question/32364656
#SPJ11
At a chemical level, alcohol addiction results in chemical changes that
A. cause the body to crave alcohol.
B. destroy all GABA receptors.
C. reduce levels of serotonin and dopamine within the brain.
D. increase cellular develop in the medulla oblongata
Answers
At a chemical level, alcohol addiction results in chemical changes that A. cause the body to crave alcohol.
Alcohol addiction can cause the body to crave alcohol. Chronic alcohol use can lead to changes in the brain's reward and pleasure pathways, affecting neurotransmitters and signaling systems involved in reward and reinforcement. These changes can result in cravings for alcohol, as the brain associates alcohol consumption with pleasurable effects.
Alcohol addiction involves complex interactions and changes within the brain and body, and the specific mechanisms and effects can vary among individuals.
You can learn more about Alcohol addiction in the link: https://brainly.com/question/12862606
#SPJ11
Be sure to answer all parts. Compounds a and b are isomers having molecular formula c5h12. Heating a with cl2 gives a single product of monohalogenation, whereas heating b under the same conditions forms three constitutional isomers. What are the structures of a and b?.
Answers
Neo-pentane represents the Compound A while compound B is n-pentane.
After careful consideration we can say that compounds A and B are alkanes and also isomers of pentane. In chemistry, Isomers are defined as compounds having same empirical molecular formula but different structural formulas due to varying arrangement of atoms.
Now, as per the question statement, compound A gives a single monochlorination product upon heating with the molecule of chlorine i.e. Cl2 showing that the molecule is extremely symmetric. This molecule must be neo-pentane. Refer to image 1.
Similarly, Compound B forms 3 constitutional isomers after undergoing monochlorination. This compound must be n-pentane since three are 3 different types of carbon atoms in the structure. Refer to image 2.
If you need to learn more about neo-pentane click here:
https://brainly.com/question/20815247
#SPJ4


Neopentane makes up component A, while n-pentane makes up compound B.
First and foremost, it is important to understand that compounds A and B are isomers and alkanes of pentane. Compounds with distinct structural formulas but the same molecular formula are known as isomers.
When heated with Cl2, compound A now produces a single monochlorination product, demonstrating the molecule's high degree of symmetry. Neopentane must be this chemical (image 1).
Upon monochlorination, compound B divides into three constitutional isomers.
A halogen atom is replaced with another substance in a process known as halogenation, where the halogen atom eventually becomes a component of the new substance or compound. In general, one or more halogens are typically added to the chemical during the halogenation reaction.
Learn more about halogens:
https://brainly.com/question/14191541
#SPJ4
what pressure will be exerted by 35.0 grams of carbon dioxide at a temperature of 25oc and a volume of 500. ml?
Answers
The pressure exerted by 35.0 grams of carbon dioxide at a temperature of 25°C and a volume of 500 ml is 3.95 atm.
The volume, pressure, temperature, and number of moles are related to one another by the ideal gas law.
The ideal gas law equation is given as follows:
P·V = n·R·T
P·V = m·RT/M, where m is the mass of the substance, and M is its molar mass. (n = m/M)
35 g of CO2 at 25°C and a volume of 500 ml can be determined as follows:
molar mass of CO2 = 44 g/moln
n = 35g / 44g/mol
n = 0.7955 moles
P·V = n·R·T
P·V = n·(0.08206 L·atm/K·mol)·T
We can now substitute the value of n and T, and the pressure can be calculated as follows:
P = n·R·T/V P
P = (0.7955 mol)·(0.08206 L·atm/K·mol)·(25°C+273.15) / 0.5 L x P
P = 3.95 atm
Therefore, the pressure exerted by 35.0 grams of carbon dioxide at a temperature of 25°C and a volume of 500. ml is 3.95 atm.
For more such questions on pressure, click on:
https://brainly.com/question/25736513
#SPJ11
following substances, which one will heat up the FASTEST? WHY?
Ammonia (4.70 J/g0C) , Aluminum (0.90 J/g0C), gold (0.13 J/g0C) , Ethylene glycol (2.42 J/g0C)
Answers
The substance that will heat up the fastest is aluminum, with a specific heat capacity of 0.90 J/g°C.
The specific heat capacity of a substance is defined as the amount of heat required to raise the temperature of 1 gram of the substance by 1°C. A lower specific heat capacity means that a smaller amount of heat is required to raise the temperature of a given amount of the substance, so the substance will heat up more quickly.
In comparison, gold has a lower specific heat capacity of 0.13 J/g°C, meaning it will heat up even faster than aluminum. Ethylene glycol has a specific heat capacity of 2.42 J/g°C, which is higher than that of aluminum, so it will take longer to heat up. Ammonia has a higher specific heat capacity of 4.70 J/g°C, meaning it will take the longest to heat up among the four substances listed.
Therefore, aluminum will heat up the fastest among these substances, due to its relatively low specific heat capacity.
Learn more about specific heat capacity:
brainly.com/question/28302909
#SPJ4
Perform the followingmathematical operation, andreport the answer to thecorrect number of significantfigures.0.97584 = 5.68 = [ ?]=Enter
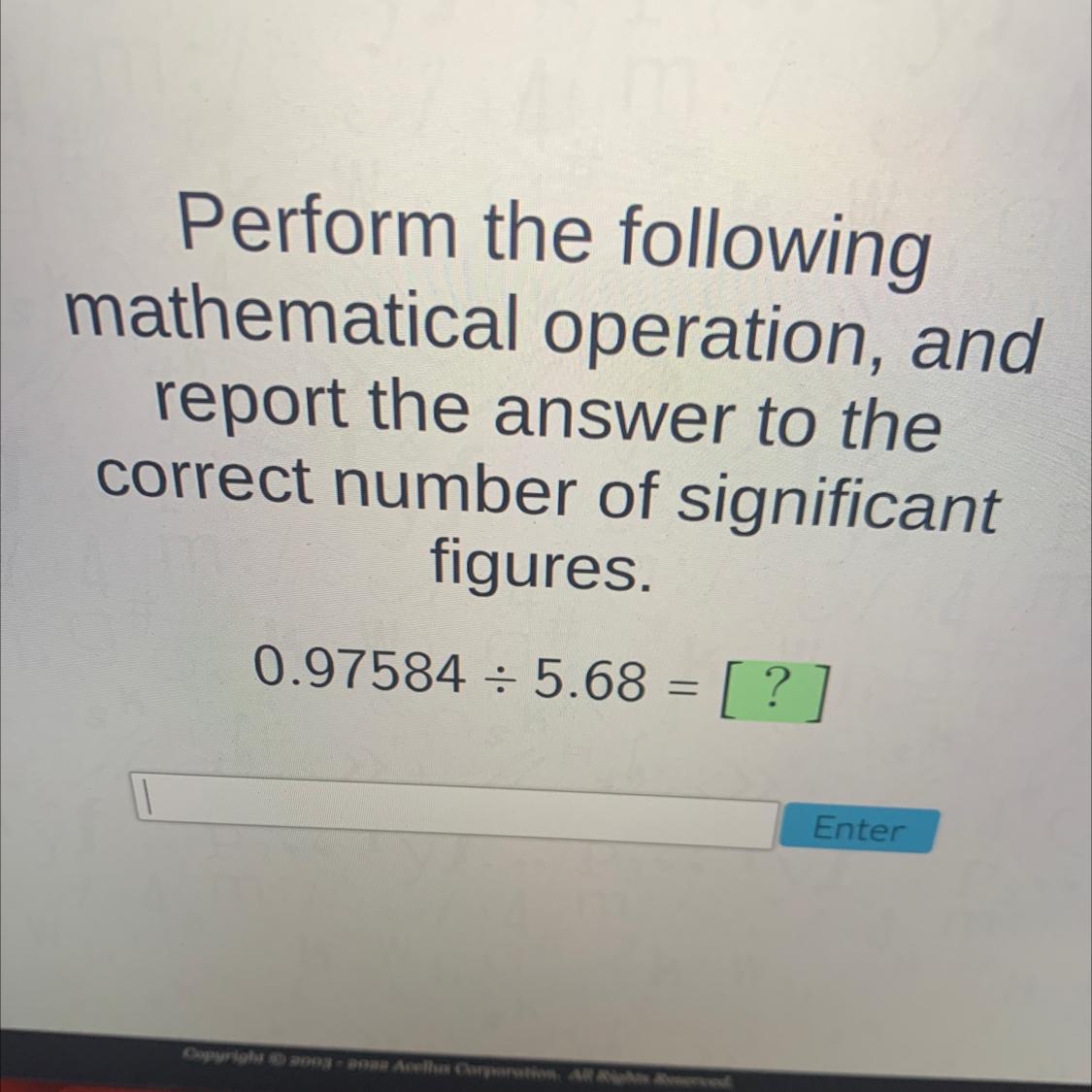
Answers
When we do the respective division operation we get the following result 0.171802.
When we do the respective division operation we get the following result 0.171802. But we must adjust the significant figures taking into account the number that has less significant figures, which is 5.68.
5.68 has three significant figures. So we will adjust the significant figures of the result to three.
0.97584 / 5.8 = 0.1718 = 0.172
Oxygen atoms (O (g), NOT O2 (g)) react with water vapor to produce hydroxyl radicals (•OH (g)). If water vapor is consumed at a rate of 1.60 x 10^-10 M/s, determine the rate of consumption of O (g).

Answers
The rate of consumption of O (g) if water vapor is consumed at a rate of 1.60 x 10⁻¹⁰ M/s is 8.0 * 10⁻⁹ M/s.
What is the rate of a reaction?The rate of a chemical reaction is known as the reaction rate or rate of reaction. It is proportional to the rise in product concentration per unit of time and the fall in reactant concentration per unit of time.
Considering the given equation:
The equation of the reaction is: O (g) + H₂O (g) ---> 2 •OH (g)
The rate of the consumption of water vapor will be twice the rate of consumption of O (g).
Water vapor is consumed at a rate of 1.60 x 10⁻¹⁰ M/s.
Hence, O (g) will be consumed at a rate of 0.5 * 1.60 x 10⁻¹⁰ M/s = 8.0 * 10⁻⁹ M/s.
Learn more about the rate of a reaction at: https://brainly.com/question/12904152
#SPJ1
i need help in science

Answers
1.
Which description accurately compares mass and weight?
A Mass indicates the density of an object, while weight indicates its volume.
B. O Mass indicates the volume of an object, while weight indicates the pull of gravity on an object.
Mass indicates how much matter an object has, while weight indicates the pull of gravity on a
Mass indicates the pull of gravity on an object, while weight indicates the amount of matter ar
D
Answers
Answer:
B
Explanation:
A stretched out rubber band is an example of:
A. kinetic energy
B. potential energy
C. gravitational energy
D. light energy
Answers
during which two processes does a substance release energy?
Answers
Answer:
Freezing and condensation
Explanation:
Because they are exothermic, they release energy
How many moles of zinc chloride will be produced when 112 grams of zinc oxide is used?
Answers
Answer:
1.38 moles
Explanation:
My teacher gave us the answer on a quiz so here if y’all want a explanation I’ll give y’all one tho
the appropriate method for removing a solvent depends on (more than one answer may be correct)
a. the volume of solvent being evaporated.
b. the yield of product.
c. the volatility of the solvent.
d. the molecular weight of the product.
e. the hazards associated with the solvent.
f. the thermal stability of the product.
Answers
The appropriate method for removing a solvent depends on several factors. The following answers are correct; volume of solvent being evaporated, volatility of the solvent, the hazards associated with the solvent, and the thermal stability of the product. Option A, C, E, and F are correct.
If a large volume of solvent needs to be removed, then a rotary evaporator or distillation may be necessary. For smaller volumes, a simple evaporation may be sufficient.
Solvents with high volatility can be removed by simple evaporation or with the help of a vacuum. Solvents with low volatility may require techniques such as rotary evaporation or distillation.
If the solvent is hazardous or toxic, special precautions such as fume hoods or closed systems may be necessary.
If the product is sensitive to heat, then techniques such as vacuum or freeze-drying may be necessary to remove the solvent.
Hence, A. C. E. F. is the correct option.
To know more about solvent here
https://brainly.com/question/14797683
#SPJ4
Topic: Mass Balance. A company sells fishmeal to be used as a protein supplement in certain foods. The process consists of: a. Extraction of fish oil, stage in which a pasta is obtained that has 20% flour and 80% water. b. Drying of pasta in a rotary drum, which produces fishmeal with 40% humidity. How much pasta must be input to the process to produce 1000 kg ?
Answers
To produce 1000 kg of fishmeal (M = 1000 kg), you would need 3000 kg of pasta. To determine the amount of pasta required to produce 1000 kg of fishmeal, we need to consider the mass balance of the process. Let's break down the steps involved:
A. Extraction of fish oil:
The pasta obtained from the extraction stage contains 20% flour and 80% water. To calculate the amount of pasta, we need to determine the mass of flour and water in the pasta. Let's assume the total mass of the pasta is P kg.
Mass of flour = 20% of P = 0.2P kg
Mass of water = 80% of P = 0.8P kg
b. Drying of pasta:
During the drying stage, the pasta is dried in a rotary drum, resulting in fishmeal with 40% humidity. This means that the final fishmeal will contain 60% dry matter.
Let's assume the mass of the dried fishmeal is M kg.
Mass of dry matter = 60% of M = 0.6M kg
Since the dry matter in the fishmeal comes from the flour in the pasta, we can equate the mass of dry matter to the mass of flour:
0.6M kg = 0.2P kg
To produce 1000 kg of fishmeal, we want to find the corresponding value of P:
0.6M = 0.2P
P = (0.6M) / 0.2
P = 3M
Therefore, to produce 1000 kg of fishmeal (M = 1000 kg), you would need 3000 kg of pasta.
To know more about mass balance, click here, https://brainly.com/question/17014679
#SPJ11
in a ________, substances have no definite volume and particles move very quickly.
Answers
Answer:
Gases
Explanation:
Particles can move quickly in gases because there is less attraction and there is more space for the particles to move.