Answers
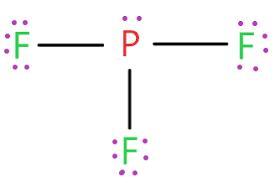
According to the concept of valence electrons and Lewis structure, the central phosphorus atom of PF3 has one nonbonding and three bonding electron pairs (s).
The electrons in an atom's highest energy levels, known as the valence electrons, are what cause interactions between atoms of the same species or of other species. That is, the valence electrons—also known as electrons in the exterior energy levels—are the electrons that will be employed in the synthesis of molecules.
In this instance
The molecule PF3, or phosphorous trifluoride, is present in this situation.
Fluorine has "7" valence electrons compared to "5" for phosphorus. Consequently, the valence electron count in phosphorous trifluoride, PF3, is 5 + 37 = 26.
The atoms that make up core atoms are often the least electronegative ones.
As a result, three P-F bonds are formed between each fluorine atom and the phosphorous atom.
Each phosphor requires one electron to complete it, but the central phosphorous atom need three to complete the octet.
As may be seen in the accompanying graphic, the Lewis structure is thus established.
You can observe that there are 20 non-bonding electrons and 6 bonding electrons in this electron-dot arrangement.
PF3's Lewis structure reveals that the core phosphorus atom contains three bonding electron pairs and one nonbonding electron pair (s).
To know more about Lewis structure, click the below link
https://brainly.com/question/29753168
#SPJ4
Related Questions
How does heat differ from temperature?
O Temperature is the measure of heat.
O Temperature and heat are the same thing.
O Temperature measures thermal energy, and heat is the flow of thermal eneroy
O Temperature measures the loss of energy, and heat measures the gain of eneray.
Answers
Answer: Temperature measures thermal energy, and heat is the flow of thermal energy
Explanation:
Heat describes the transfer of thermal energy between molecules within a system. Heat measures how energy moves or flows. It is measured in Joules.
Temperature measures the hotmess or coldness of a body or how much thermal energy it contains . Temperature describes the average kinetic energy of molecules within a material or system. It is measured in Celsius (°C), Kelvin(K), or Fahrenheit (°F).
Thus the correct option is Temperature measures thermal energy, and heat is the flow of thermal energy
Answer:
its c
Explanation:
edg 2021
Calculate the mass of calcium carbonate produced if 15.2 mL of 0.306 M K2CO3 is reacted with 17.0 mL of 0.295 M CaCl2.
Answers
Answer:
\(m_{CaCO_3}=0.465g\)
Explanation:
Hello!
In this case, since the reaction between two aqueous solutions may turn out in the production of a solid precipitate, for potassium carbonate and calcium chloride, calcium carbonate is precipitated out as shown below:
\(CaCl_2(aq)+K_2CO_3(aq)\rightarrow 2KCl(aq)+CaCO_3(s)\)
Now, since the two reactants are in a 1:1 mole ratio, we infer they react in the same proportion, thus we compute the reacting moles, considering the used volumes of those molar solutions:
\(n_{K_2CO_3}=0.0152L*0.306mol/L=0.00465mol\\\\n_{CaCl_2}=0.0170*0.295mol/L=0.00502mol\)
Thus, since just 0.00465 mol out of 0.00502 moles of calcium chloride are consumed, the potassium carbonate is the limiting reactant, therefore the mass of yielded calcium carbonate (molar mass = 100.09 g/mol) is:
\(m_{CaCO_3}=0.00465molK_2CO_3*\frac{1molCaCO_3}{1molK_2CO_3} *\frac{100.09gCaCO_3}{1molCaCO_3}\\\\m_{CaCO_3}=0.465g\)
Best regards!
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
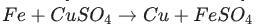
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
What is the pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C? A) 4.48 x 10¹¹ atm B) 2.24 x 10⁰ atm C) 1.12 x 10³ atm D) 2.24 x 10³ atm
Answers
The pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C is 2.24 × 10⁰ atm.
How to calculate pressure?The pressure of a substance can be calculated using the following formula;
PV = nRT
P = pressureV = volumen = no of molesR = gas law constantT = temperatureAccording to this question, the pressure, in atmospheres, exerted by a 0.100 mol sample of oxygen in a 2.00 L container at 273 °C can be calculated as follows:
P × 2 = 0.1 × 0.0821 × 546
2P = 4.48266
P = 2.24 × 10⁰ atm
Learn more about pressure at: https://brainly.com/question/31525061
#SPJ1
please help if you know all 4

Answers
Answer:
1. lobster
3. Two parts of skeleton meet.
Giant planet atmospheres have layers of clouds and aerosols (tiny liquid droplets) made from different chemicals because:
A) convection does not occur on giant planets.
B) the Coriolis effect affects each chemical compound differently.
C) different chemicals condense at different temperatures.
D) the winds are in the outermost layer.
Answers
Matter are anything that is made up of atoms. The quantity of matter can be observed only on the basis of mass and volume calculation. Thus option C is correct option.
What is matter?Matter is a substance that has some mass and can occupy some volume. The matter is mainly used in science. Matter can be solid, liquid or gas.
Matter is anything that is made up of atoms. Anything around us that can be physically seen and touched are matter. Ice, water and water vapors are example of matter.
Giant planet atmospheres have layers of clouds and aerosols (tiny liquid droplets) made from different chemicals because different chemicals condense at different temperatures.
Therefore, option C is correct option.
To learn more about matter, here:
https://brainly.com/question/4562319
#SPJ1
The mass number of Fe2+ is 56. How many neutrons are there in a single Fe2+ atom?
28
30
56
58
Answers
Answer:
A.) 28
Explanation:
The mass number is the number of protons and neutrons within a particular atom. The charge of the atom is irrelevant because the mass number is not affected by the number of electrons.
Unless this atom is an isotope (different number of neutrons), there should be an equal amount of protons and neutrons.
As such, since the mass number is 56, there should be 28 protons and 28 neutrons (56 / 2 = 28).
50.0 mL each of 1.0 M HCl and 1.0 M NaOH, at room temperature (20.0 OC) are mixed. The temperature of the resulting NaCl solution increases to 27.5 OC. The density of the resulting NaCl solution is 1.02 g/mL. The specific heat of the resulting NaCl solution is 4.06 J/g OC Calculate the Heat of Neutralization of HCl(aq) and NaOH(aq) in KJ/mol NaCl produced
Answers
Answer:
-62.12kJ/mol is heat of neutralization
Explanation:
The neutralization reaction of HCl and NaOH is:
HCl + NaOH → NaCl + H₂O + HEAT
An acid that reacts with a base producing a salt and water
You can find the released heat of the reaction -heat of neutralization- (Released heat per mole of reaction) using the formula:
Q = C×m×ΔT
Where Q is heat, C specific heat of the solution (4.06J/gºC), m its mass of the solution and ΔT change in temperature (27.5ºC-20.0ºC = 7.5ºC).
The mass of the solution can be found with the volume of the solution (50.0mL of HCl solution + 50.0mL of NaOH solution = 100.0mL) and its density (1.02g/mL), as follows:
100.0mL × (1.02g / mL) = 102g of solution.
Replacing, heat produced in the reaction was:
Q = C×m×ΔT
Q = 4.06J/gºC×102g×7.5ºC
Q = 3106J = 3.106kJ of heat are released.
There are 50.0mL ×1M = 50.0mmoles = 0.0500 moles of HCl and NaOH that reacts releasing 3.106kJ of heat. That means heat of neutralization is:
3.106kJ / 0.0500mol of reaction =
-62.12kJ/mol is heat of neutralizationThe - is because heat is released, absorbed heat has a + sign
What do we mean when we say a substance is "pure"?
Answers
Answer:
A pure substance is a substance that is only made up of atoms of one element and so it has fixed melting and boiling points.
In a Lewis Diagram for fluorine, are the two electrons given to the other fluorine atom, taken by the other fluorine atom, or shared between the fluorine atoms?
Answers
Enter your answer in the provided box. Acrylic acid (CH2═CHCOOH) is used to prepare polymers, adhesives, and paints. The first step to make acrylic acid involves the vapor-phase oxidation of propylene (CH2═CHCH3) to acrolein (CH2═CHCHO). This step is carried out at 330°C and 2.50 atm in a large bundle of tubes around which circulates a heat-transfer agent. The reactants spend an average of 1.80 s in the tubes, which have a void space of 92.1 ft3. How many pounds of propylene must be added per hour in a mixture whose mole fractions are 0.0700 propylene, 0.3500 steam, and 0.5800 air?
Answers
Answer: m = 1710.35 pounds per hour
Explanation: Since the step involves a gaseous state, it can be used the ideal gas formula, given by:
PV=nRT
For this question, R will be 8.31451 m³Pa/K.mol, which means the variables need a change of unit:
T = 330°C + 273 = 603K
P = 2.5atm*101325 = 253312.5Pa
V = 92.1ft³*0.0283 = 2.60643m³
Calculating mols:
PV=nRT
\(n=\frac{253312.5*2.60643}{8.31451*603}\)
n = 131.7 mols
The reactant (propylene) spends 1.80 seconds in the tubes and 1 hour is 3600 seconds, so:
\(\frac{131.7*3600}{1.8}\) = 263377.5 mol/h
In the mixture, the proportion of propylene is 0.07, then:
263377.5*0.07 = 18436.4 mol of propylene in the mixture
Mol is related to mass and molar mass by the following formula:
\(n=\frac{m}{M}\)
m = nM
Molar Mass of propylene is 42.08g/mol:
m = 18436.4*42.08 = 775803.712g
Changing into pounds:
\(m=\frac{775803.712}{453.6}\)
m = 1710.35lbs
It must be added 1710.35lbs per hour in the mixture.
Balance the equation and indicate whether it is a synthesis reaction, a decomposition reaction, a single-displacer
CHÚCO Xe Hyon Coa
Balanced equation
Type of reaction e
a. Double-displacement
b. 2CH/a)+0)-2H₂O(g)+CO₂(g)
c. Decomposition
d. Synthesis
e. Combustion
1.CH/a)+20/a)-H₂O(g)+200-(a)
g Single-displacement
h. CHg) 20(g)-2H₂O(g)+CO₂(g)
Answers
4.00 g of O2 gas are in a sealed, 2.00 L gas canister at 22.0 °C what is the pressure inside this container (in atm)?
Answers
Answer:
1.51448 atms
Explanation:

The unknown ionic compound gave a green flame test, precipitate with ammonium carbonate, and a colorless/light yellow halide test. What is the unknown compound?
Answers
Answer:
BaI2
Explanation:
Barium gives a green flame test. When the halide test is conducted, a yellow precipitate indicates the presence of the iodide ion.
Also, BaI2 reacts with ammonium carbonate as shown below:
BaI2(aq) + (NH4)2CO3(aq) → BaCO3(s) + 2(NH4)I(aq)
The BaCO3(s) separates from the reaction system as a precipitate.
helppp nowwww plsss

Answers
Answer:
i think its 3
Explanation:
because cloud are made from water vapor and clouds also got preciptation if im wrong sorry
17. HAZWOPER training and certification recognizes:
a. A large number (as much as 80%) will self-present or be self-referred victims
b. Awareness level training will promote proper initial triage actions
c.
Victims will use any entrance they can enter at the hospital, in addition to the
emergency department entrance
d. Both A and C
Answers
HAZWOPER training and certification recognize:
a large number (as much as 80%) will self-present or be self-referred victimsVictims will use any entrance they can enter at the hospital, in addition to the emergency department entranceThe correct option is both A and C
What is the HAZWOPER training and certification?HAZWOPER (Hazardous Waste Operations and Emergency Response) training and certification recognize that a large number of victims (as much as 80%) in hazardous waste incidents or emergencies will self-present or be self-referred for medical treatment.
Additionally, HAZWOPER training acknowledges that victims may use any entrance they can access at a hospital, not just the emergency department entrance.
This is because individuals affected by hazardous materials may arrive at different areas of the hospital seeking medical assistance.
Therefore, option d. Both A and C are correct statements regarding the recognition of HAZWOPER training and certification.
Learn more about HAZWOPER at: https://brainly.com/question/31561828
#SPJ1
5. An example of a muscle that is voluntarily controlled is a muscle that
A. Makes the leg move.
B. Makes the heart pump.
C. Squeezes blood through vessels.
D. Squeezes food in the stomach.
Answers
Answer:
A, Makes the leg move
Explanation:
What is the molecule shown below?н син н Н НТТТТТТн-с-с-с-с-с-с-н||||||H H CHOH Н НO A. OctaneOB. 4-propylpentaneOC. 2,3-dimethylhexaneOD. 2-pentylpropane

Answers
This molecule in the question presents 6 Carbons in its main chain, counting only the carbons in the middle, which makes it a Hexane molecule. Besides the main chain carbons, we also have two methyl groups linked to carbons 2 and 3, counting from left to right. Therefore the final name will be:
2,3-dimethylhexane. Letter C
I need to mark the steepest areas.

Answers
The hilltops and the steepest point of a slope are found in the highest and most closely-spaced contour lines on the map.
What are contour lines?Contour lines are lines on a map that connect points of equal elevation. Each contour line represents a specific elevation, and the spacing between contour lines represents the slope of the terrain.
The closer the contour lines are to each other, the steeper the slope. The hilltops are found where the contour lines form a closed circle or oval shape. The steepest point of a slope is found where the contour lines are closest together, indicating the highest rate of change in elevation.
Learn more about contour lines at: https://brainly.com/question/13088900
#SPJ1
For an experiment, you make a solution that is 0.500 M CaCl₂(aq). The
correct molar concentration for Ca(aq) and Cl(aq) in solution is?
Answers
The term molarity is an important method which is used to calculate the concentration of a solution. It is also known as the term molar concentration. Here molarity is given as 0.500 M.
The concept molarity is defined as the number of moles of the solute present per liter of the solution. It is represented as 'M' and its unit is mol / L. Let us take the volume as 20 L.
Then molarity = Number of moles / Volume in Liters
n = 20 × 0.500 = 10
Molar concentration of 'Ca' = 10 / 20 = 0.5
Molar concentration of 'Cl' = 2 × 0.5 = 1
To know more about molarity, visit;
https://brainly.com/question/16727614
#SPJ1
Chlorophyll allows plants to carry out photosynthesis. There are two forms of chlorophyll:
chlorophyll al ( C55H7205N, Mg) and chlorophyll 6 ( C55H7006N, Mg). What is the
difference in molar mass between these two forms?
Answers
Answer:
Chlorophyll-a/b-carotenoid complexes, known as light harvesting complexes, protect photosynthetic system from strong light. Chlorophylls are insoluble in water due to the absence of polar groups.
Explanation:
Hope this helps :)
Maintenance of............of the body such as temperature level, water content, pH and blood pressure to be in balanced and stable condition
Answers
People who are infected with HIV can infect others:
A. If they develop opportunistic infections
B. Only after they have had an HIV test.
C. After they become infected even if they look and feel healthy.
D. Only when they have symptoms
Answers
Answer:
C. After they become infected even if they look and feel healthy.
Explanation:
HIV is a virus and even when you don't have symptoms, if you have it, you can still infect others. Not taking an HIV test doesn't mean you don't have it, it just means that you don't know that you have it. And I don't think an opportunistic infection would really change the affect of a virus spreading.
A virus can spread no matter the circumstances, it doesn't care what type of person you are or what background you have; and it's kind of like cancer in that way.
Write a short essay about life in the Han Dynasty, comparing it to life today. Make sure to include key features:
-Family
-Government
-Social Structure
-Religion
-Trade
Answers
Answer:
Life in the Han Dynasty (206 BCE - 220 CE) differed significantly from today in family, government, social structure, religion, and trade. For example, the Han Dynasty emphasized a patriarchal family structure, where the eldest male held authority, and filial piety was highly valued. In contrast, contemporary societies embrace more egalitarian family dynamics with shared decision-making.
The government system of the Han Dynasty relied on a centralized bureaucracy and emphasized meritocracy, while modern societies often adopted democratic systems. Socially, the Han Dynasty followed a hierarchical model influenced by Confucian principles, whereas contemporary societies strive for greater equality and social mobility.
Religion in the Han Dynasty combined Confucianism, Taoism, and Buddhism, whereas modern societies exhibit diverse religious beliefs. Lastly, trade in the Han Dynasty thrived along the Silk Road, while modern trade was globally interconnected and facilitated by technological advancements. These differences highlight the evolution of society over time.
Explanation:
How many grams of Aspirin (acetylsalicylic acid) should we form in this reaction if we started with 2.08g of Salicyclic Acid?
Answers
Taking into account the reaction stoichiometry, 2.713 grams of aspirin are formed when 2.08 grams of salicyclic acid reacts.
Reaction stoichiometryIn first place, the balanced reaction is:
C₄H₆O₃ + C₇H₆O₃ (Salicyclic Acid) →C₉H₈O₄ (aspirin) + C₂H₄O₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
C₄H₆O₃: 1 moleC₇H₆O₃: 1 moleC₉H₈O₄: 1 moleC₂H₄O₂: 1 moleThe molar mass of the compounds is:
C₄H₆O₃: 102 g/moleC₇H₆O₃: 138 g/moleC₉H₈O₄: 180 g/moleC₂H₄O₂: 60 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
C₄H₆O₃: 1 mole ×102 g/mole= 102 gramsC₇H₆O₃: 1 mole ×138 g/mole= 138 gramsC₉H₈O₄: 1 mole ×180 g/mole= 180 gramsC₂H₄O₂: 1 mole ×60 g/mole= 60 gramsMass of aspirin formedThe following rule of three can be applied: if by reaction stoichiometry 138 grams of salicyclic acid form 180 grams of aspirin, 2.08 grams of salicyclic acid form how much mass of aspirin?
\(mass of aspirin=\frac{2.08 grams of salicyclic acidx 180 grams of aspirin}{138 grams of salicyclic acid}\)
mass of aspirin= 2.713 grams
Then, 2.713 grams of aspirin are formed when 2.08 grams of salicyclic acid reacts.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
amyelkin22 Yesterday Chemistry College consider a solution containing alcohol and water. If the mole fraction of water is 0.600, what is the mole fraction of alcohol?
Answers
A solution containing alcohol and water. If the mole fraction of water is 0.600, 0.4 is the mole fraction of alcohol.
What is mole fraction?A mole fraction is a measurement of concentration that is equal to the product of the moles of a component and the total moles of the solution.
Mole fraction is indeed a unitless phrase since it represents a ratio. When all the parts of a solution's mole fraction are summed up, they equal one.
moles fraction of water + mole fraction of alcohol =1
0.600+ mole fraction of alcohol =1
mole fraction of alcohol= 1-0.600
=0.4
Therefore, 0.4 is the mole fraction of alcohol.
To know more about mole fraction, here:
https://brainly.com/question/29808190
#SPJ1
Which part of the landscape shown in this image is the steepest?

Answers
Answer: A I believe
Explanation:
Use your data, the equation to the right, and the specific heat of water (4.184 J/g C) to compute the specific heat values of each metal. Use a calculator and round to the nearest hundredth place.
Answers
The heat capacity for the metals are;
Aluminum - 0.89
Copper - 0.11
Iron - 0.44
Lead - 0.12
What is the specific heat?The specific heat of a substance is denoted by the symbol "C" and is typically measured in units of J/g·°C (joules per gram per degree Celsius) or cal/g·°C (calories per gram per degree Celsius).
The specific h
We have that;
For Aluminum;
c = 4.184 * 39.85 * 4.7/11.98 * 72.9
= 783.6/873.3
= 0.89
For Copper;
c = 4.184 * 12.14 * 1.9/12.14 * 75.4
= 96.5/915.3
= 0.11
For Iron
c = 4.184 * 40.24 * 2.4/12.31 * 75.1
= 404.1/924.5
= 0.44
For Lead
c = 4.184 * 39.65 * 0.7/12.46 * 76.7
c = 116.1/955.68
= 0.12
Learn more about specific heat:https://brainly.com/question/31608647
#SPJ1

How many moles of NaCl can be produced from 2.5 moles of BaCl_2.
Answers
2.5 moles of BaCl2 can produce 5 moles of NaCl.
What is chemical equation?
Chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas.
The balanced chemical equation for the reaction between barium chloride (BaCl2) and sodium sulfate (Na2SO4) is:
BaCl2 + Na2SO4 → 2 NaCl + BaSO4
From this equation, we can see that for every 1 mole of BaCl2, 2 moles of NaCl are produced.
Therefore, to calculate how many moles of NaCl can be produced from 2.5 moles of BaCl2, we can use the following proportion:
1 mole BaCl2 : 2 moles NaCl = 2.5 moles BaCl2 : x moles NaCl
Where
x is the number of moles of NaCl producedSolving for x, we get:
x = 2.5 moles BaCl2 × (2 moles NaCl / 1 mole BaCl2)
x = 5 moles NaCl
Therefore, 2.5 moles of BaCl2 can produce 5 moles of NaCl.
Learn more about chemical equation here : brainly.com/question/26694427
#SPJ1
What is the formula for Pentasulfur heptaoxide
Answers
Answer:
S5O7
Explanation: