the influence of matrix and laser energy on the molecular mass distribution of synthetic polymers obtained by maldi-tof-ms. int. j. mass spectrom. 2004, 238(3), 215–
Answers
The article discusses the impact of matrix and laser energy on the molecular mass distribution analysis of synthetic polymers using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF-MS).
In MALDI-TOF-MS, a laser is used to ionize and desorb analyte molecules from a sample matrix, allowing their detection and analysis. This technique is particularly useful for characterizing synthetic polymers as it provides information about their molecular weight distribution.
The article explores how the choice of matrix and laser energy can affect the accuracy and precision of the obtained molecular mass distribution. The matrix refers to the material that is mixed with the polymer sample before analysis to facilitate ionization and enhance signal intensity. Different matrices have varying effects on the ionization efficiency and fragmentation patterns of polymer molecules, which can impact the observed mass distribution.
Additionally, the laser energy used during the ionization process can influence the extent of fragmentation and ionization efficiency. Higher laser energies may cause more extensive fragmentation, leading to the generation of smaller fragments and potentially affecting the observed molecular mass distribution.
The study likely investigates how different combinations of matrix materials and laser energy settings can be optimized to obtain accurate and representative molecular mass distributions of synthetic polymers. By understanding these factors, researchers can improve the reliability and reproducibility of MALDI-TOF-MS analysis for polymer characterization.
learn more about molecular mass here
https://brainly.com/question/31476184
#SPJ11
Related Questions
When you dissolve 1 mole of hbr in enough water to make 1 l solution, you expect the resulting solution to have: ________
Answers
When you dissolve 1 mole of hydrobromic acid (HBr) in enough water to make a 1 liter solution, you expect the resulting solution to have a concentration of 1 Molar (1 M) HBr.
A 1 M solution of HBr is an aqueous solution, meaning that it contains HBr ions in the form of H+(aq) and Br-(aq) in water. The concentration of HBr in the solution is expressed in moles per liter (M), where 1 M represents 1 mole of solute (HBr) per liter of solution.
In this case, since 1 mole of HBr is dissolved in enough water to make 1 liter of solution, the concentration of the HBr solution will be 1 M.
Hydrobromic acid (HBr) is a strong acid, meaning that it dissociates completely in water to form hydrogen ions (H+) and bromide ions (Br-). The resulting solution will have a high concentration of H+ ions and a corresponding low pH, typically around 1.
The concentration of HBr in a solution can be calculated using the equation:
C = n/V
where C is the concentration of the solution in moles per liter (M), n is the number of moles of solute (HBr), and V is the volume of the solution in liters.
Learn more about concentration here:
https://brainly.com/question/10725862
#SPJ4
which of th efollwong is not a technique to prevent drowsy driving
Answers
The technique that is not effective in preventing drowsy driving is listening to loud music.
While listening to music may help some drivers stay alert, listening to loud music can actually be distracting and increase fatigue. The other techniques for preventing drowsy driving include getting enough sleep, taking breaks every two hours, drinking caffeine, and driving with a passenger who can help keep the driver alert.
In summary, while listening to music can be helpful in preventing drowsy driving, it is important to avoid listening to music too loudly. It is important to use other techniques such as getting enough sleep, taking breaks, drinking caffeine, and having a passenger to help prevent drowsy driving.
learn more about drowsy driving
https://brainly.com/question/30243513
#SPJ11
what compound has a molar mass of 37.11 g/mol
Answers
Answer:
Explanation:
The closest I can come to this is
Li C2H5 which would really be weird, but it comes to nearly 37.11. Everything depends on the periodic table you are using.
C2 = 2 * 12.011 = 24.022
H5 = 5 * 1.008 = 5.040
Li = 1 * 6.94 6.94
Total 36.002
in the movie star wars episode v: the empire strikes back, the character han solo is frozen in the fictional material, carbonite. if carbonite existed, what would be its likely chemical formula?
Answers
If carbonite existed, its likely chemical formula will be CO₂²⁻.
The carbonite is liquid substance that is made from the carbon and the oxygen. the carbonite could change in the solid form through rapid freezing. carbonite is used to freeze the good for preservation is called as the carbon freezing. the carbonite is the blasting explosive. so, the carbon freezing is the process in which the liquid carbonite is frozen into the solid state.
Thus, in the movie star wars episode v: the empire strikes back, the character han solo is frozen in the fictional material, carbonite. if carbonite existed, its likely chemical formula will be CO₂²⁻.
To learn more about carbonite here
https://brainly.com/question/29596527
#SPJ4
Explain why [H, 0] is not included in the calculation of the K of the borax (see Equation 5 page 138). 2. A 9.00 mL aliquot of a borax-borate equilibrium solution reacts complete- ly with 29.10 mL of a 0.100 M HCl solution. Calculate the K, of the borax. 3. From the parameters of the best-fit line, determine AH and AS. Be sure to report the correct units for these quantities. What does the fit, R2, tell you about your graph and the values of AH and AS determined? к- [NEBOCH,1 (5)
Answers
The reason why [H, 0] is not included in the calculation of the K of borax is that it is not a significant contributor to the overall equilibrium of the system.
Borax, or sodium borate, reacts with HCl to form a complex ion, so the equilibrium equation only involves the concentrations of borax and the complex ion.
To calculate the K of the borax, we can use the equation;
K = [complex ion]/[borax]
Here, first, the determination of the concentration of the complex ion is required which is done by using the volume and concentration of the HCl solution that reacts with the borax-borate equilibrium solution.
Later, the equation n = C x V is used to determine the amount of HCl that reacts, then use stoichiometry to determine the amount of complex ion that is formed.
The moles of HCl reacted: (29.10 mL)(0.100 M) = 2.910 mmol.
Since there's a 1:1 ratio between HCl and borate, 2.910 mmol of borate reacted.
Thus, the initial concentration of borate is (2.910 mmol)/(9.00 mL) = 0.323 M.
To determine ΔH and ΔS, plot the graph of ln(K) vs 1/T and find the slope and y-intercept of the line of best fit.
Here, the slope is equal to -ΔH/R and the y-intercept is equal to ΔS/R, where R is the gas constant.
The units for ΔH are J/mol and the units for ΔS are J/(mol*K).
The value of R² tells us how well the data points fit the line of best fit.
A value of 1 means that all data points lie on the line, while a value of 0 means that none fit the line.
The closer R² is to 1, the more confident one can be in the values of ΔH and ΔS that are determined.
To know more about borax-borate concentration, click below.
https://brainly.com/question/21133994
#SPJ11
Using standard absolute entropies at 298K, calculate the entropy change for the system when 1.68 moles of CO(g) react at standard conditions.
Consider the reaction
CO(g) + H2O(l)CO2(g) + H2(g)
Using standard absolute entropies at 298K, calculate the entropy change for the system when 1.68 moles of CO(g) react at standard conditions.
Answers
The entropy change for the system when 1.68 moles of CO(g) react at standard conditions is 180.2 J K-1.
The entropy change (ΔS) for the given reaction can be calculated by using the standard absolute entropies of each species at 298 K. The balanced chemical equation for the given reaction is CO(g) + H2O(l) → CO2(g) + H2(g).
The molar absolute entropy of CO(g), H2O(l), CO2(g), and H2(g) are 197.9 J K-1 mol-1, 69.9 J K-1 mol-1, 213.6 J K-1 mol-1, and 130.7 J K-1 mol-1 respectively. The entropy change for the given reaction can be calculated as:
ΔS = ΣnS(products) - ΣnS(reactants)
ΔS = (1 mol x 213.6 J K-1 mol-1) + (1 mol x 130.7 J K-1 mol-1) - (1.68 mol x 197.9 J K-1 mol-1) - (1 mol x 69.9 J K-1 mol-1)
ΔS = 360.12 J K-1 - 333.012 J K-1
ΔS = 27.088 J K-1
Therefore, the entropy changes for the system when 1.68 moles of CO(g) react at standard conditions is 27.088 J K-1 or 180.2 J K-1 mol-1 (calculated by dividing by the number of moles of CO(g)).
Know more about entropy, here:
https://brainly.com/question/20166134
#SPJ11
which element is reduced in this reaction? cr2o72− + 3 hno2 + 5 h+ → 2 cr3+ + 3 no3− + 4 h2o
Answers
The reaction is: Cr2O7^2- + 3 HNO2 + 5 H+ → 2 Cr^3+ + 3 NO3^- + 4 H2O. To identify the element that is reduced, we need to analyze the change in oxidation states of the elements involved:
1. Chromium (Cr) changes from Cr2O7^2- to Cr^3+. In Cr2O7^2-, Cr has an oxidation state of +6, and in Cr^3+, it has an oxidation state of +3. This means the oxidation state has decreased.
2. Nitrogen (N) changes from HNO2 to NO3^-. In HNO2, N has an oxidation state of +3, and in NO3^-, it has an oxidation state of +5. This means the oxidation state has increased.
Since reduction involves a decrease in oxidation state, Chromium (Cr) is the element that is reduced in this reaction, changing from Cr2O7^2- to Cr^3+.
Learn more about oxidation states here:
brainly.com/question/31688257
#SPJ11
1. List 3 physical and chemical phenomena
Answers
Answer:
Examples of chemical changes are burning, cooking, rusting, and rotting. Examples of physical changes are boiling, melting, freezing, and shredding. Often, physical changes can be undone, if energy is input.
Hope this helped.
thylbenzene is heated in a container at 1.5 atm until 20% of the original liquid is evaporated. determine the composition of the vapor and liquid phases and find the operating temperature. (3 pts.)
Answers
When methylbenzene is heated in a container at 1.5 atm until 20% of the original liquid is evaporated, the composition of the vapor and liquid phases can be determined using Raoult's Law. The operating temperature can also be found using the Antoine equation.
First, let's determine the composition of the vapor phase. According to Raoult's Law, the partial pressure of a component in the vapor phase is equal to the mole fraction of that component in the liquid phase multiplied by the vapor pressure of the pure component at the given temperature.
PA = xA PA*
Where PA is the partial pressure of component A in the vapor phase, xA is the mole fraction of component A in the liquid phase, and PA* is the vapor pressure of pure component A at the given temperature.
Since 20% of the original liquid has evaporated, the mole fraction of methylbenzene in the liquid phase is 0.8. The vapor pressure of pure methylbenzene at the given temperature can be found using the Antoine equation:
log10PA* = A - B/(T + C)
Where PA* is the vapor pressure of pure component A at the given temperature, T is the temperature in degrees Celsius, and A, B, and C are constants.
Using the Antoine equation and the values of A, B, and C for methylbenzene, we can solve for the temperature and find the operating temperature.
Once we have the vapor pressure of pure methylbenzene and the mole fraction of methylbenzene in the liquid phase, we can use Raoult's Law to find the partial pressure of methylbenzene in the vapor phase.
The composition of the liquid phase will be 80% methylbenzene and 20% of the other components. The composition of the vapor phase will be the partial pressure of methylbenzene divided by the total pressure (1.5 atm).
Therefore, the composition of the vapor and liquid phases and the operating temperature can be determined using Raoult's Law and the Antoine equation.
To know more about vapor phase visit:
https://brainly.com/question/72632#
#SPJ11
A body is accelerated continuously. What is the form of the graph?
A. cubic
B. inverse
C. linear
D. quadratic

Answers
Answer:
B: Inverse
Explanation:
The dog has a mass of 57kg and the boy has a mass of 48 kg. Who has more kinetic energy?

Answers
Answer:
The Dog
Explanation:
The more mass something has the more kinetic energy it has in it.
The volume of a sample of pure HCl gas was 221 mL at 20°C and 111 mmHg. It was completely dissolved in about 50 mL of water and titrated with an NaOH solution; 18.7 mL of the NaOH solution was required to neutralize the HCl. Calculate the molarity of the NaOH solution.
Answers
Answer:
\(molarity =6.9\times 10^{-3}\ M\\\)
Explanation:
We know that , the reaction of HCl and NaOH is given as follows
\(NaOH+HCl=NaCl +H_2O\)
Given that
Pressure = 111 mm Hg
\(P=111\times 13.6\times 10^{-3}\times 9.81\times 1000=14.809\ kPa\)
Temperature = 20°C
T=20+273=293 K
Volume= 221 m L
V=0.221 L
Number of moles of HCl is given as follows
\(n=\dfrac{P\times V}{R\times T}\\n=\dfrac{0.148\times 0.221}{0.821\times 293}=1.3\times 10^{-4}\ moles\)
From the above reaction we can say that
Number of moles of HCl=Number of moles of NaOH
Volume of NaoH is given as follows
V=18.7 = 0.0187 L
Therefore molarity
\(molarity =\dfrac{n}{V_{NaOH}}\\molarity =\dfrac{1.3\times 10^{-4}}{0.0187}=6.9\times 10^{-3}\ M\\molarity =6.9\times 10^{-3}\ M\\\)
.a new rose dust is being prepared by using two available products: pest and bug. each kilogram of pest contains 30 g of carbaryl and 40 g of malathion, whereas each kilogram of bug contains 40 g of carbaryl and 20 g of malathion. the final blend must contain at least 120 g of carbaryl and at most 80 g of malathion. if each kilogram of pest costs $3.00 and each kilogram of bug costs $2.50, how many kilograms of each pesticide should be used to minimize the cost?
Answers
To minimize the cost of the final blend, you should use 2 kg of PEST and 1 kg of BUG. This would provide 120 g of carbaryl and 80 g of Malathion, which meets the required amounts, while keeping the cost at $7.50 ($3.00 for 2 kg of PEST and $2.50 for 1 kg of BUG).
To ensure the effectiveness of the rose dust, it is important to understand the properties of both pesticides and their effects on the environment. Carbaryl is a contact insecticide, meaning it works by coming into contact with the insect and killing it.
Learn more about pesticide :
https://brainly.com/question/6589507
#SPJ4
write the molecular and net ionix versions of the reaction of aluminum bromide and mercury (II) nitrate
Answers
To find the speed of the piton just before striking the ground, we can use the formula for gravitational potential energy:
PE = mgh
Where m is the mass of the piton (41.5 g or 0.0415 kg), g is the acceleration due to gravity (9.8 m/s^2), and h is the height from which the piton was dropped (355 m).
So, the potential energy of the piton at the top of the cliff is:
PE = (0.0415 kg) x (9.8 m/s^2) x (355 m) = 138.9 J
At the bottom of the cliff, all of this potential energy will have been converted into kinetic energy, or the energy of motion. So we can use the formula for kinetic energy to find the speed of the piton:
KE = 1/2mv^2
Where KE is the kinetic energy, m is the mass of the piton, and v is its speed.
Setting KE equal to the potential energy we just calculated, we can solve for v:
1/2 (0.0415 kg) v^2 = 138.9 J
v^2 = (2 x 138.9 J) / 0.0415 kgv^2 = 106,024 m^2/s^2
v = sqrt(106,024) = 325.5 m/s
So the speed of the piton just before striking the ground would be approximately 325.5 m/s, assuming no air resistance.
To know more about potential energy visit:-
https://brainly.com/question/24284560
#SPJ11
Write the equation for the equilibrium contant, K, for the binding of oxygen to hemoglobin
Answers
Equilibrium constant K for the reaction can be written as,
K = [C]c [D]d / [A]a [B]b
In the absence of oxygen, cells cannot carry out their biochemical responsibilities. Oxygen moves to the cells attached to hemoglobin. Equilibrium constant is the value of its reaction quotient. The equilibrium constant, K, expresses the relationship between products and reactants of a reaction at equilibrium with respect to a specific unit. The equilibrium constant K is the ratio of the mathematical product of the concentrations of the products of a reaction to the mathematical product of the concentrations of the reactants of the reaction. protein hemoglobin which binds oxygen in your lungs and carries it to other tissues of your body. hemoglobin bind oxygen tightly so that a large fraction of the hemoglobin will pick up an oxygen before cycling back through the other tissues. However, in the other tissues, one wants hemoglobin to bind oxygen loosely so that it can be easily relinquished for use in respiration. Each concentration is raised to the power of its coefficient in the balanced chemical equation.
suppose a general reaction written,
aA + bB---> cC + dD
For the reaction equilibrium constant can be written as,
Keq = [C]c [D]d / [A]a [B]b
The reaction of equilibrium of hemoglobin is,
Hb(CO)4 + 4O2 ⇌ Hb(O2)4 + 4CO
To know more about Equilibrium constant please visit :
https://brainly.com/question/3159758
#SPJ4
Total these measurements. Your answer should indicate the proper accuracy.
28 L
42 L
6 L
931 L
____
Your answer should indicate the proper precision (correct number of insignificant figures).
1. 1,010 L
2. 1,007.0 L
3. 1,007 L
4. 1,000 L
Answers
Answer:
The sum of given measurements is 1007 L.
Explanation:
The given measurements are 28 L, 42 L, 6 L, and 931 L.
We need to find the total of these measurements.
Total = 28 L + 42 L + 6 L + 931 L
It can be written as
Total = ( 28 +42+6+931)
One simplification we get Total = 1007 L
The volume of 3. 70 moles of a gas is 47. 2 L at a certain temperature and pressure. At the same temperature and pressure, the moles of gas that occupies 25. 5 L
Answers
Approximately 2.00 moles of gas would occupy 25.5 L at the same temperature and pressure.
To find the number of moles of gas that occupies 25.5 L at the same temperature and pressure, we can use the principle of molar volume. Molar volume is the volume occupied by one mole of any gas at a specific temperature and pressure.
Given that 3.70 moles of gas occupies 47.2 L, we can set up a proportion to find the moles of gas that occupies 25.5 L:
(3.70 moles) / (47.2 L) = x / (25.5 L)
Solving for x, we can cross-multiply and divide:
x = (3.70 moles * 25.5 L) / 47.2 L
x ≈ 2.00 moles
In order to determine the number of moles of gas occupying 25.5 L at the same temperature and pressure, we need additional information regarding the temperature and pressure values. The volume of a gas is directly influenced by temperature and pressure according to the ideal gas law equation: PV = nRT, where P represents pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T stands for temperature.
Learn more about temperature and pressure here:
https://brainly.com/question/30313321
#SPJ11
25 points!!! For the following equation, give the number you would use in the mole-to-mole ratio for each of the reactants and products as well as the number you would use in the volume-to-volume
ratio for each of the gaseous reactants and products. Check to make sure the equation is balanced.
CH4(g) + O2(g) → CO2(g) + H2O (g)
Reactant/Product
CH₂(g)
O₂ (g)
CO₂(g)
H₂O(g)
Mole-to-Mole Number
Volume-to-Volume Number
If you know the volume of substance A, how would you solve for the volume of substance B? Please list the steps you would take in the
correct order.

Answers
For the given equation, the mole-to-mole ratio and volume-to-volume ratio for all the gaseous reactants and products are 1:1.
For the given equation:
CH4(g) + O2(g) → CO2(g) + H2O(g)
Reactant/Product:
CH4(g)
O2(g)
CO2(g)
H2O(g)
Mole-to-Mole Ratio:
By comparing the reactant and product coefficients, we may extract the mole-to-mole ratio from the balanced equation.
CH4(g): 1 mole
O2(g): 1 mole
CO2(g): 1 mole
H2O(g): 1 mole
All of the reactants and products have a mole-to-mole ratio of 1:1:1:1.
Ratio of Volume to Volume:
The ideal gas law, which states that under constant temperature and pressure, the volume of a gas is directly proportional to the number of moles in the gas, can be used to calculate the volume-to-volume ratio.
All gaseous reactants and products have a mole-to-mole ratio of 1:1, hence the volume-to-volume ratio will also be 1:1. As a result, the volume of drug A and substance B will be equal.
You can just take the volume of substance A as the value for substance B if you know the volume of substance A and are trying to solve for the volume of substance B. Alternatively, the quantity of substances A and Bwill be the same, given the 1:1 volume-to-volume ratio.
For more such questions on reactants visit:
https://brainly.com/question/26283409
#SPJ8
Which of the following are true about Earth’s axis. Choose the two correct answers.
A a line that passes through Earth’s equator
B a line around which Earth rotates
C a line along which Earth travels around the sun
D a line that passes through Earth’s North and South Poles
E a line from Earth's surface to its center
Answers
Answer:
B and D
Explanation:
the first step in the preparation of magnesium metal is the precipitation of mg(oh)2 from sea water by the addition of ca(oh)2. the concentration of mg2 (aq) in sea water is 5.37 × 10–2 m. calculate the ph at which [mg2 ] is decreased to 1.0 × 10–5 m
Answers
The first step in the preparation of magnesium metal is the precipitation of Mg(OH)₂ from sea water by the addition of Ca(OH)₂ the concentration of Mg₂ (aq) in sea water is 5.37 × 10⁻² m then ph at which [Mg₂ ] is decreased to 1.0 × 10⁻⁵ m is 1.27
Magnesium is the element and also one of the alkaline earth metal and metallic magnesium is prepared either by electrolysis of molten MgCl₂ or by metallothermic reduction of its halides by alkali or alkaline-earth metals
Here given data is concentration = 5.37 × 10⁻² m
We have to find pH= ?
pH = -log[HA]
pH = -log[Mg₂]
pH = -log[5.37 × 10⁻² m]
pH = 1.27
Know more about pH
https://brainly.com/question/22312484
#SPJ1
my answer was 10 and it’s telling me it’s wrong..what did i do wrong?
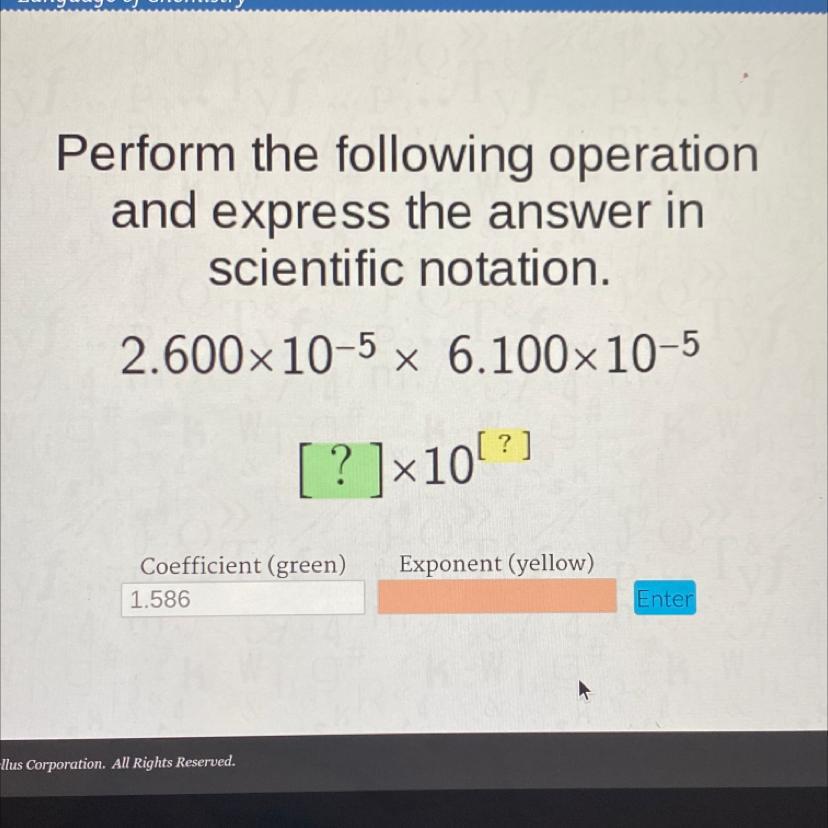
Answers
Answer:
1.586x10^-9
Explanation:
To make a multiplication in scientific notation we need to multiply the coefficients and sum the exponents:
Coefficients: 2.600 * 6.1000 = 15.86
Exponents: -5 + (-5) = -10
The result is:
15.86x10^-10
As the scientific notation must be given with only 1 number in the left of the point:
1.586x10^-9In an ionic compound, the size of the ions affects the internuclear distance (the distance between the centers of adjacent ions), which affects lattice energy (a measure of the attractive force holding those ions together).
Based on ion sizes, rank these compounds of their expected lattice energy.
Note: Many sources define lattice energies as negative values. Rank by magnitude and ignore the sign.
Lattice energy = absolute value of the lattice energy.
Greatest |lattice energy| (strongest bond)
least |lattice energy| (strongest bond)
MgBr_2, MgF_2, MgCl_2, MgI_2
Answers
The compounds ranked by their expected lattice energy from greatest to least are: MgF_2, MgCl_2, MgBr_2, MgI_2.
Lattice energy is a measure of the energy released when gaseous ions come together to form an ionic solid. It is influenced by factors such as ion charge and ion size. In general, as the charges of the ions increase, the lattice energy also increases. However, when comparing ions with the same charge, the size of the ions becomes the determining factor.
In the given compounds, the common ion is Mg_2+ (with a +2 charge), while the anions are F-, Cl-, Br-, and I-. Among these anions, fluoride (F-) has the smallest ionic radius, followed by chloride (Cl-), bromide (Br-), and iodide (I-). Smaller ions have a higher charge density, meaning the positive charge is concentrated in a smaller space, leading to stronger attractive forces between the ions.
Therefore, based on ion size, the compound with the greatest expected lattice energy is MgF_2, followed by MgCl_2, MgBr_2, and MgI_2, with MgF_2 having the strongest bond and MgI_2 having the weakest bond.
Learn more about lattice energy from the given link:
https://brainly.com/question/29735933
#SPJ11
the rate of reaction would also increase if the temperature increases why
Answers
Answer:
An increase in temperature typically increases the rate of reaction. An increase in temperature will raise the average kinetic energy of the reactant molecules. Therefore, a greater proportion of molecules will have the minimum energy necessary for an effective collision (Figure.
Explanation:
Hope it helps
In the following reaction, how many grams of silver can be produced from 49.1 g copper?
Cu(s) + 2AgNO3(aq) ® 2Ag(s) + Cu(NO3)2(aq)
Answers
166.4 g Ag grams of silver can be produced from 49.1 g of copper.
What is a mole?A mole is a very important unit of measurement that chemists use. A mole of something means you have 602,214,076,000,000,000,000,000 of that thing, like how having a dozen eggs means you have twelve eggs.
\(1 mol Cu + 2 mol AgNO_3\) → \(2 mol Ag + 1 mol Cu(NO3)2\)
63.55 g Cu —> 2 x 107.688 g Ag
63.55 g Cu gives 215.376 g of Ag
So, 49.1 g Cu —> \(\frac{215.376 g X 49.1 g}{63.55 g}\)
= 166.4 g Ag
Hence, 166.4 g Ag grams of silver can be produced from 49.1 g of copper.
Learn more about moles here:
https://brainly.com/question/26416088
#SPJ1
At 20 °C, a sample of copper occupying a volume of 8.50 cmº has a mass of 75.6
grams. What is the density of the copper?
Answers
Answer:
The answer is 8.89 g/cm³Explanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question
mass = 75.6 g
volume of copper = 8.5 cm³
We have
\(density = \frac{75.6}{8.50} \\ = 8.894117647...\)
We have the final answer as
8.89 g/cm³Hope this helps you
What two of the following organisms are secondary consumers in this food web?
Answers
Secondary consumers are organisms that primarily feed on herbivores or other primary consumers.
They occupy the next trophic level above the primary consumers in a food web. They obtain energy by consuming the primary consumers and play an important role in regulating the population of herbivores.
Examples of commonly observed secondary consumers include:
Carnivorous mammals: Animals such as wolves, lions, and tigers that feed on herbivores like deer, zebras, or gazelles.
Birds of prey: Species like eagles, hawks, and owls that consume small mammals, reptiles, or other birds.
Carnivorous fish: Fish like pike, barracuda, or bass that prey on smaller fish or aquatic invertebrates.
Predatory insects: Insects such as spiders, mantises, or dragonflies that feed on other insects, including herbivorous insects.
In a specific food web, the identification of secondary consumers would depend on the specific organisms present and their feeding interactions. It would be necessary to analyze the trophic relationships among the organisms in the food web to determine the secondary consumers accurately.
For more such questions on Secondary consumers visit:
https://brainly.com/question/28631974
#SPJ8
What point were the authors of the Declaration trying to make in the Preamble?
HELP.
Answers
Answer:
To explain why they are revolting against the king
Explanation:
Which statement describes the particles in a gas?
Choose the correct answer.
Gas particles are spaced far apart and move around freely.
Gas particles are spaced far apart and move around slowly.
Gas particles are tightly packed together and move past each other freely.
Gas particles are loosely packed together and move past each other freely.
Answers
Gas particles are spaced far apart and move around freely is the statement that describes the particles in a gas Option A is correct.
What is gas?The gas is the state of matter in which the particles of matter are vey far away from each other and are not even visible from the normal human eyes and can move in zig zag motion in the environment.
As the particles are very much spaced from each other so the shape of the gas is also not fixed it can only be felt not can be seen from the eyes and particles are independent and free to move.
Therefore, Option A is correct. Gas particles are spaced far apart and move around freely is the statement that describes the particles in a gas.
Learn more about gas, here:
https://brainly.com/question/14812509
#SPJ2
how many moles of nh3 would be produced if 6 moles of h2 reacted with 3 moles of n2?
Answers
The chemical equation for the reaction of hydrogen gas (H2) and nitrogen gas (N2) to form ammonia gas (NH3) is as follows N2 + 3H2 → 2NH3 3 moles of hydrogen gas .
react with 1 mole of nitrogen gas to form 2 moles of ammonia gas, according to this equation. As a result, we can use a mole ratio to calculate the number of moles of ammonia created when 6 moles of hydrogen react with 3 moles of nitrogen 6 moles H2 x (2 moles NH3/ 3 moles H2) = 4 moles NH3. As a result, if 6 moles of H2 react with 3 moles of N2, 4 moles of NH3 are formed. The chemical equation for the reaction of hydrogen gas (H2) and nitrogen gas (N2) to form ammonia gas (NH3) is as follows N2 + 3H2 → 2NH3 3 moles of hydrogen gas .
learn more about moles here:
https://brainly.com/question/26416088
#SPJ4
what is the iupac name for the following compound? a. e-2-heptenol b. e-2-heptanol c. z-2-heptenol d. z-2-heptanol
Answers
The IUPAC name for the following compound (attached as a picture) is (a) e-2-heptenol.
First, we see whether it is a heptanol or heptenol, so if any alkyl group has a double bond we call it an alkene, And without a double bond, we call it an alkane, "en" and "an" are signs for alkene, and alkane respectively. As in the given compound, there is a double bond so it is heptenol not heptanol. Now find out it is E or Z, as we can see that higher priority groups are on the opposite sides of the double bond. So it is clear that the given compound is e-2-heptenol.
You can also learn about IUPAC naming from the following question:
https://brainly.com/question/16631447
#SPJ4
