the half-life of radium-226 is 1620 years. how long will it take for the original amount to be reduced by 55%?
Answers
If the half-life of radium-226 is 1620 years. The for 55% decomposition of its original amount will take 1397 years.
Calculate the half-life by using the formula as,
A(t) = I(0.5)^t/t1/2
Where t1/2 = half life = 1620
Final amount, A(t) = 0.55 of its original amount = 0.55I
Hence, we have
0.55I = I(0.5)^t/1620
Taking the log of both sides we get,
log(0.55) = log(0.5)^t/1620
t/1620 = log(0.55) / log(0.5)
t/1620 = 0.8624964
t = 0.8624964 × 1620
t = 1397.2441
t = 1397 (nearest whole number)
Hence, for 55% decomposition of its original amount will take 1397 years.
Learn more about half life from the link given below.
https://brainly.com/question/14883322
#SPJ4
Related Questions
A substance has a density of 0.0835 g/mL. What is its density in kilograms per cubic meter (kg/m³)?
Answers
Answer:
83.5 kg/m^3
Explanation:
One g/mL is 1000 kg/m^3 so just multiply it by 1000.
Which quantity is the same before and after a nuclear change? (1 point)
O atomic mass of each atom
O atomic number of each atom
O total mass
O total charge
Answers
The atomic number of each atom is the same before and after a nuclear change
What is an atom?An particle is the fundamental building square of matter. It is the littlest unit of an component that holds the chemical properties of that component. Particles are unimaginably modest and composed of indeed littler particles.
In a atomic alter, such as radioactive rot or atomic fission/fusion, the core of an atom experiences a change. This may include the discharge of particles, the part of the core, or the combination of cores. In any case, the atomic number of an component, which speaks to the number of protons within the core, remains steady all through any atomic alter.
Learn about atomic number here https://brainly.com/question/11353462
#SPJ1
Help meeeeeeeeeeeeeee
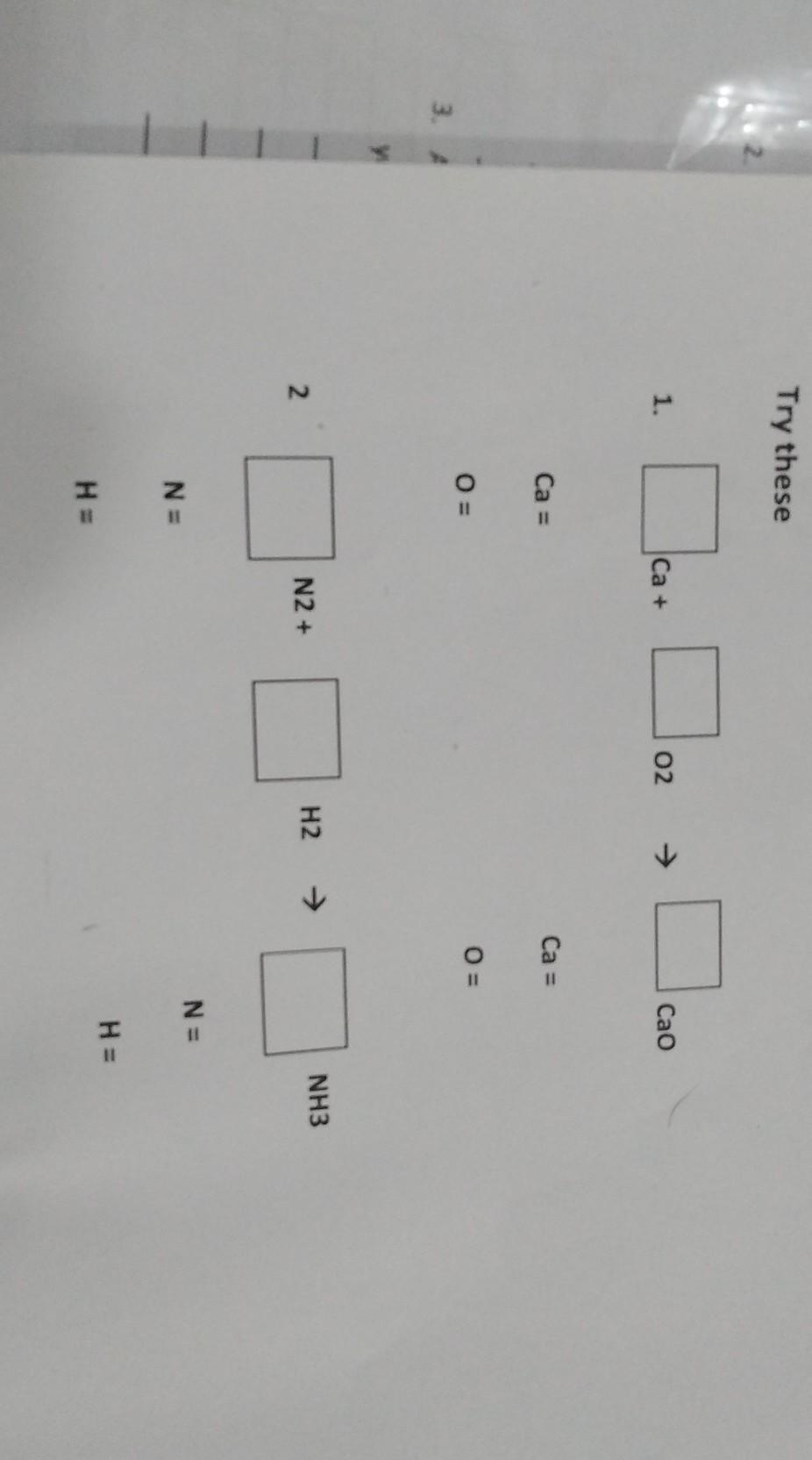
Answers

Among neutral (uncharged) organic compounds: _________________ forms one covalent bond and no unshared pairs of electrons.
Answers
Among neutral organic compounds, the group of compounds known as alkanes typically form one covalent bond and have no unshared pairs of electrons.
Alkanes are characterized by having a general formula of CnH2n+2, where n is the number of carbon atoms in the molecule.
The carbon atoms in alkanes are each bonded to four other atoms, typically hydrogen atoms, resulting in a tetrahedral geometry and no unshared pairs of electrons.
learn more about hydrogen here :
https://brainly.com/question/28937951
#SPJ11
Fluorine, chlorine, and iodine are examples of
Answers
Answer:
Halogens
Explanation:
The halogens are a series of non-metal elements from group 17 of the periodic table (formerly VII). The halogens include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
Answer:
halogens
Explanation:
just did the test on edg!
can periodic table indicates occurrance of the elements, why?
Answers
Answer: No
Explanation:
A periodic table can be defined as the table in which the row of chemical elements are present. All the elements of the row have same number of electron shells.
The arrangement of elements is based on atomic numbers, electron shell model and other chemical properties. The elements are placed in increasing order of atomic number.
This table does not give information about the occurrence of the elements.
first, alex dose an exprerimen wich she fills 2 cups with cold liquid at the same tempeture: 1 with the new lid she finds that the liquid in one cup warmed up more than the liquid in the other cup after 10 minuts wich cup do yo think it wermed up more why
Answers
The temperature of liquid in a cup with a lid changes less than in a cup without a lid.
How does a lid affect the temperature of liquid inside the cup?It is well known that in a closed system, neither heat nor matter will be transferred from the system to the environment. In contrast, in an open system, energy and heat are exchanged from the system to its surroundings and vice versa.
Cup with the lid (cup A) is a closed system in this situation, but Cup without lid (cup B) is an open system. Therefore, there won't be any heat or material transfer from cup A to the system or surroundings. As a result, cup B warm up slowly.
We can conclude that cup with lids are able to keep liquid cool better than the cup without the lids. Therefore, after 10 minutes the liquid in the cup without lid will be more warmed up.
Know more about how lid affects the liquid in a cup at:
https://brainly.com/question/12702491
#SPJ1
How many kiloliters are in 96
milliliters?
Answers
Answer:
\(9.6e - 5\)
this is the answer to that problem
Given parameters:
Volume = 96 milliliters
Problem;
Convert into kiloliters
These are volume units which represent the amount of space a body occupies. We use volume for a solid body and fluids.
We are to convert milliliters to kiloliters;
milli- and kilo- are prefixes
milli = \(\frac{1}{1000}\) kilo = 1000
Since the standard of comparison is liters, let us establish the relationship;
1000mL = 1L
1000L = 1kL
where mL denotes milliters and kL denotes kiloliters;
Now we can solve;
Since
1000mL = 1L
96mL = x
1000x = 96
x = \(\frac{96}{1000}\) = 0.096L
Now we convert this volume to kL, kiloliters;
1000L = 1KL
0.096L = x
1000x = 0.096
x = \(\frac{0.096}{1000}\) = 9.6 x 10⁻⁵kL
Therefore in 96mL equals 9.6 x 10⁻⁵kL
will an electron and a proton attract or repel one another? how about two electrons?
Answers
Electron and proton attract each other.
Two electrons will repel each other.
An electron is a negatively charged subatomic particle that can be either sure to an atom or free (no longer certain). An electron this is certain to an atom is one of the three primary varieties of particles inside the atom -- the other are protons and neutrons. collectively, electrons, protons and neutrons shape an atom's nucleus.
A proton is a subatomic particle observed inside the nucleus of each atom. The particle has a positive electric price, same and opposite to that of the electron. If remoted, a single proton could have a mass of best 1.673 ? 10-27 kilogram, simply barely much less than the mass of a neutron.
Learn more about Electron here:-https://brainly.com/question/860094
#SPJ4
Which layer of the soil profile would be affected the most by weathering and erosion?
A 1
B 2
C 3
D4
Please help

Answers
Answer:
A1
Explanation:
Why is it usually not possible to recrystallize a liquid substance? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a. liquids have too much kinetic energy b. liquids transition to gases easier than to solids c. liquids have high potential energy d. liquids at room temperature do not readily transition into solids or crystals e. all of the above
Answers
Liquids at room temperature do not readily transition into solids or crystals (Option D) is why is it usually not possible to recrystallize a liquid substance.
Why is it usually not possible to recrystallize a liquid substance?Recrystallization involves dissolving a solid in a suitable solvent and then allowing it to cool and reform into crystals. However, liquids do not readily form crystals at room temperature because their molecules are disordered and lack the organized arrangement necessary for crystal formation.
Additionally, liquids may have insufficient intermolecular forces to hold the crystal lattice together. Therefore, recrystallizing a liquid substance is usually not possible.
Learn about Recrystallization here https://brainly.com/question/10194206
#SPJ1
What are the units of molar mass?
A. L/g
B. mol/g
C. g/L
D. g/mol
SUBST
Answers
Answer:
D - g/mol
step by step method
I think its the best answer
the volume of a sample of hydrogen gas at 0.997 atm is 5.00l. what will be the new volume if the pressure is decreased to 0.977 atm?
Answers
The new volume of the hydrogen gas sample, when the pressure is decreased from 0.997 atm to 0.977 atm, can be calculated using Boyle's law. The new volume will be approximately 5.10 L.
Boyle's law states that at a constant temperature, the volume of a gas is inversely proportional to its pressure. Mathematically, this relationship can be expressed as:
\(\[ P_1 \cdot V_1 = P_2 \cdot V_2 \]\)
where \(\( P_1 \)\) and \(\( V_1 \)\) are the initial pressure and volume, and \(\( P_2 \)\) and \(\( V_2 \)\) are the final pressure and volume.
Given that the initial pressure \((\( P_1 \))\) is 0.997 atm and the initial volume \((\( V_1 \))\) is 5.00 L, and the final pressure \((\( P_2 \))\) is 0.977 atm, we can solve for the final volume \((\( V_2 \))\):
\(\[ P_1 \cdot V_1 = P_2 \cdot V_2 \]\)
\(\[ 0.997 \, \text{atm} \cdot 5.00 \, \text{L} = 0.977 \, \text{atm} \cdot V_2 \]\)
Solving for \(\( V_2 \)\):
\(\[ V_2 = \frac{{0.997 \, \text{atm} \cdot 5.00 \, \text{L}}}{{0.977 \, \text{atm}}} \approx 5.10 \, \text{L} \]\)
Therefore, the new volume of the hydrogen gas sample, when the pressure is decreased to 0.977 atm, will be approximately 5.10 L.
To learn more about Boyle's law refer:
https://brainly.com/question/1696010
#SPJ11
a liquid mixture of one or more substances that cannot be separated by filtering or allowing the mixture to stand is called a/an:
Answers
A homogeneous mixture, also known as a solution, is a liquid mixture where one or more substances are combined in such a way that they cannot be separated by filtering or allowing the mixture to stand. Unlike heterogeneous mixtures, such as suspensions or colloids, homogeneous mixtures have uniform composition and properties throughout.
The different substances in a homogeneous mixture are evenly distributed at a molecular or atomic level, resulting in a single phase.
This uniform distribution makes it challenging to visually differentiate the individual components of the mixture.
Examples of homogeneous liquid mixtures include saltwater, vinegar, and ethanol-water solutions.
The inability to separate the components of a homogeneous mixture by simple physical means is due to the intimate mixing and molecular interactions between the substances involved.
Read more about Liquid mixture.
https://brainly.com/question/12160179
#SPJ11
A learner was assigning oxidation numbers for different elements in the compounds OF2 and NaF. The learner assigned F an oxidation number of 1+ in OF2 and -1 in NaF.
Is this correct? Why?
A. Yes this is correct
B. No, fluorine is always assigned an oxidation number of -1.
C. No, fluorine is always assigned an oxidation number of +1.
Answers
Answer: B. No, fluorine is always assigned an oxidation number of -1.
Explanation:
True or false: plants, animals, and the environment are not dependent on each other for the use of carbon and oxygen.
Answers
They basically just swap oxygen for CO2, or CO2 for oxygen! So the answer is false because they DO depend on each other for the use of Oxygen and Carbon dioxide! Hope this helped :)
What formula represents the compound formed from barium and hypochlorite?
Answers
Answer:
Ba(ClO)₂
Explanation:
The compound formed from barium and hypochlorite is barium hypochlorite, and its chemical formula is Ba(ClO)₂.
A chemical element with the atomic number 56 is barium (Ba). It belongs to the alkaline earth metal family and when it forms compounds, it has a 2+ charge.
This indicates that two electrons must be rapidly lost by barium in order to produce a stable electron configuration.
One chlorine atom (Cl) and one oxygen atom (O₂) combine to form the anion hypochlorite (ClO-) (O). The hypochlorite ion has a net charge of -1 due to the 1-charged oxygen atom and the 1-charged chlorine atom.
The 2+ charge of barium interacts with the 1- charge of the hypochlorite ion throughout the reaction to create a neutral molecule.
To know more about barium hypochlorite:
https://brainly.com/question/26845679
#SPJ6
Why is Hydrogen on Alkali Metal side?
Answers
Hydrogen is an Alkali Metal side because it has ns1 electron configuration like the alkali metals. elements are very reactive
If a gas at 25.0 °C occupies 3.60 liters at a pressure of 1.00 atm, what will be its volume at a pressure of 2.50 atm?
Answers
Answer:
1.44 L
Explanation:
Since 25 is constant it is no use. Now, rearrange the gas formula. You should get...
P1V1/T2=P2V2T1
Next, rearrange to fit the problem. You should get...
V2=P1V1/P2
Fill in our values and solve. You should get 1.44 L
We can check this by knowing that P and V at constant T have an inverse relationship. Hence, this is correct.
- Hope that helps! Please let me know if you need further explanation.
PLS HELP i will give 10 points
In Mendeleev’s Periodic Table, why was there no mention of Noble gases like Helium, Neon and Argon?
Answers
Answer:Answer choices are:
A. These elements did not fit in with his pattern.
B. These elements are gases and he didn't include gases.
C. The atoms of these elements had no atomic mass.
D. These elements had not yet been discovered.
___________________________________________________________
Correct answer choice is:
D. These elements had not yet been discovered.
what is being reduced? in the electrochemical cell: zn(s) | zn2 (aq) || ag (aq) | ag(s) what is being reduced? ag (aq) ag(s) zn2 (aq) zn(s)
Answers
In the given electrochemical cell: Zn(s) | Zn²⁺(aq) || Ag⁺(aq) | Ag(s), silver (Ag) is being reduced. The reaction for the same can be represented as follows: Ag⁺(aq) + e⁻ → Ag(s).
Here, silver ion (Ag⁺) is gaining an electron (e⁻) to form silver metal (Ag) which is a reduction process. Therefore, the answer is "Ag (aq) and Ag(s)" which means silver is being reduced in the given electrochemical cell.
In chemistry, an electrochemical cell refers to a system that uses chemical reactions to produce electric current or harness electrical energy to drive chemical reaction and it consists of two electrodes (an anode and a cathode) immersed in an electrolyte solution.
To know more about electrochemical cell, refer
https://brainly.com/question/25749323
#SPJ11
How to balance this equation with algebraic method ( please include explanation )
KNO3 + H2CO3 = K2CO3 + HNO3
Answers
Answer:
(ANSWER)
2 KNO3 + H2CO3 = K2CO3 +2 HNO3 is the balanced equation.
PA BRAINLYES
enough of a monoprotic acid is dissolved in water to produce a 1.33 m solution. the ph of the resulting solution is 2.64 . calculate the ka for the acid.
Answers
The Ka of the monoprotic acid is 3.98 × 10⁻³.
The pH of a solution can be related to the acid dissociation constant (Ka) of an acid by the following equation;
pH = pKa + log([A⁻]/[HA]),
where [A⁻] is the concentration of the conjugate base and [HA] is the concentration of the acid. Since the acid is monoprotic, [A⁻] is equal to the concentration of acid that has dissociated, and [HA] is equal to the initial concentration of the acid.
We can start by calculating the initial concentration of the acid from the given molarity of the solution;
1.33 mol/L = [HA]
Next, we can use the pH of the solution to calculate the concentration of the conjugate base;
pH = 2.64 = -log[H⁺]
[H⁺] = \(10^{-2.64}\) = 3.98 × 10⁻³ mol/L
[OH⁻] = 1.00 × 10⁻¹⁴ / [H⁺] = 2.51 × 10⁻¹² mol/L
[OH⁻] [HA] / [A⁻] = Kw = 1.00 × 10⁻¹⁴ mol²/L²
[HA] = [A⁻] + [H⁺]
Assume that [HA] = [A⁻] since the acid is weak, and the dissociation is small compared to the initial concentration of the acid
[HA] = [A⁻] = 1.33 mol/L
Substituting these values into the pH equation and solving for Ka gives;
2.64 = pKa + log([A⁻]/[HA])
2.64 = pKa + log(1/1)
pKa = 2.64
Now, we can use the definition of Ka to calculate its value:
Ka = [H⁺][A⁻]/[HA]
Ka = (3.98 × 10⁻³ mol/L) (1.33 mol/L) / (1.33 mol/L)
Ka = 3.98 × 10⁻³
To know more about monoprotic acid here
https://brainly.com/question/2515115
#SPJ4
A solution is made by combining 15.0mL of 18.5M acetic acid with 5.60g of sodium acetate and diluting to a total volume of 1.50 L.
Calculate the pH of the solution.
Answers
The pH of the solution is approximately 4.75.This indicates that the solution is slightly acidic.
To calculate the pH of the solution, we need to determine the concentration of acetate ions and acetic acid. First, let's find the number of moles of sodium acetate:
Mass of sodium acetate = 5.60 g
Molar mass of sodium acetate (CH3COONa) = 82.03 g/mol
Number of moles of sodium acetate = 5.60 g / 82.03 g/mol = 0.068 mol
Next, we need to find the number of moles of acetic acid:
Volume of acetic acid = 15.0 mL = 0.015 L
Concentration of acetic acid = 18.5 M
Number of moles of acetic acid = 18.5 mol/L * 0.015 L = 0.278 mol
Now, we can calculate the total volume of the solution:
Total volume = 1.50 L
The total moles of acetate ions can be calculated by summing the moles of sodium acetate and acetic acid:
Total moles of acetate ions = 0.068 mol + 0.278 mol = 0.346 mol
Now, we calculate the molarity (M) of the acetate ions:
Molarity of acetate ions = Total moles of acetate ions / Total volume
= 0.346 mol / 1.50 L = 0.231 M
Since sodium acetate is a strong electrolyte, it will dissociate completely in water, providing an equal concentration of acetate ions (0.231 M). The concentration of acetic acid is 0.278 M (determined earlier).
The Henderson-Hasselbalch equation can be used to calculate the pH of the solution:
pH = pKa + log([Acetate]/[Acetic Acid])
The pKa of acetic acid is 4.76.
pH = 4.76 + log(0.231/0.278)
≈ 4.75
The pH of the solution is approximately 4.75. This indicates that the solution is slightly acidic. The calculation involved determining the concentrations of acetate ions and acetic acid in the solution and using the Henderson-Hasselbalch equation to calculate the pH.
To know more about pH visit :
https://brainly.com/question/12609985
#SPJ11
HELP PLZ
Write a Scientific Explanation, minimum of 5 paragraphs: What makes weather change, what cause storms? Why is weather different from place to place?
Answers
Answer:
Scientific Explanation of weather change and cause storms is described below in detail.
Explanation:
The weather is just the nature of the environment at any time, including items such as warmth, rainfall, air pressure, and cloud shelter. Daily variations in the weather are due to storms and winds. Seasonal variations are due to the Earth spinning nearby the sun. Warm beginnings often produce stormy weather as the heated air mass at the exterior rises above the cool air mass, creating clouds and storms.
CO2 + H2O --> C2H2 + O2
Answers
Answer:
C2H2 + 5 O2 = 4 CO2 + 2 H2O
Add / Edited: 27.09.2014 / 25.01.2015
Evaluation of information: 5.0 out of 5 / number of votes: 2
Source: https://chemiday.com/en/reaction/3-1-0-339
Explanation:
Calculate each of the following quantities.
(a) total number of ions in 47.8 g of srf2
(b) mass (kg) of 4.90 mol of cucl2 · 2 h2o
(c) mass (mg) of 2.67 1022 formula units of bi(no3)3 · 5 h2o
Answers
There are 4.59 × 10²³ ions in 47.8 g of SrF₂.
The mass of 4.90 mol of CuCl₂ · 2H₂O is 0.83495 kg.
The mass of 2.67 × 10²² formula units of Bi(NO₃)₃ · 5H₂O is 1.30 × 10³⁴ mg.
(a) The molar mass of SrF₂ is 125.62 g/mol. Thus, there are 0.380 moles of SrF₂ in 47.8 g. Since each formula unit of SrF₂ produces two ions (Sr²⁺ and 2F⁻), the total number of ions can be calculated by multiplying the number of formula units by the number of ions per formula unit:
0.380 mol SrF₂ × 6.02 × 10²³ formula units/mol × 2 ions/formula unit = 4.59 × 10²³ ions
As a result, there are 4.59 × 10²³ ions in 47.8 g of SrF₂.
(b) The molar mass of CuCl₂ · 2H₂O is 170.48 g/mol. The mass of 4.90 mol of CuCl₂ · 2H₂O can be calculated by multiplying the molar mass by the number of moles:
4.90 mol × 170.48 g/mol = 834.95 g
Since there are 1000 g in 1 kg, 4.90 mol of CuCl₂ · 2H₂O weighs 0.83495 kilogram.
(c) The molar mass of Bi(NO₃)₃ · 5H₂O is 485.09 g/mol. The mass of 2.67 × 10²² formula units of Bi(NO₃)₃ · 5H₂O can be calculated by multiplying the molar mass by the number of formula units:
2.67 × 10²² formula units × 485.09 g/mol = 1.30 × 10²⁷ g
Since there are 10⁶ mg in 1 g, 1.30 × 10³⁴ mg is the mass of 2.67 × 10²² formula units of Bi(NO₃)₃ · 5H₂O.
To know more about the Mass, here
https://brainly.com/question/27190103
#SPJ4
A sample of natural sulfur consists of three isotopes:
95.0% sulfur-32 (31.97 amu)
0.75% sulfur-33 (32.97 amu)
4.21% sulfur-34 (33.96 amu)
Based on this information, what is the average molar mass of sulfur?
Answers
The average molar mass of sulfur is 32.84g/mol
The average atomic mass of a chemical element is calculated by taking into account the atomic masses of its naturally occurring isotopes and their respective abundances
Here given data is natural sulfur consists of three isotopes :
95.0% sulfur-32 = 31.97 amu
0.75% sulfur-33 = 32.97 amu
4.21% sulfur-34 = 33.96 amu
So, we have to find average molar mass of the sulfur = ?
Formula for average molar mass = ∑i × isotope i × abundance i
³²S = 95.0% abundance = 31.97 amu
³³S = 0.75% abundance = 32.97 amu
³⁴S = 4.21% abundance = 33.96 amu
This means that average molar mass of sulfur is
Average molar mass = 31.97 amu×95.0 + 32.97 amu× 0.75 + 33.96 amu×4.21
Average molar mass = 32.84g/mol
Average molar mass of sulfur is 32.84g/mol
Know more about average molar mass
https://brainly.com/question/20910101
#SPJ1
A 3.5L flexible container holds a gas at 250K. What will the new volume be if the temperature is increased to 400K?
Answers
Answer: 5.6L
Explanation:
Just did it on an assignment
A 3.5L flexible container holds a gas at 250K. The new volume will be 5.6 L, if the temperature is increased to 400K.
What is Charles Law ?Charles law is an ideal gas law which states that at a constant pressure volume of a gas is directly proportional to the absolute temperature.
It is expressed as
\(\frac{V_1}{T_1} = \frac{V_2}{T_2}\)
where,
V₁ = initial volume
T₁ = initial temperature
V₂ = final temperature
T₂ = final temperature
Now put the values in above formula, we get
\(\frac{V_1}{T_1} = \frac{V_2}{T_2}\)
\(\frac{3.5\ L}{250\ K} = \frac{V_2}{400\ K}\)
\(V_{2} = \frac{3.5\ L \times 400\ K}{250\ K}\)
\(V_{2} = \frac{1400}{250}\)
V₂ = 5.6 L
Thus, from the above conclusion we can say that A 3.5L flexible container holds a gas at 250K. The new volume will be 5.6 L, if the temperature is increased to 400K.
Learn more about the Charles Law here: https://brainly.com/question/16927784
#SPJ2
Susie cooked sausages on a barbecue. Fat and water in the sausages changed state. which two changes of state occur during this process? a. Fat melted b. Water evaporated
Answers
Answer:
The options a and b are correct
Explanation:
This options provided to the question are the answers to the question. But for clarification. When the fat melts, the change of state that occurs here is from solid to liquid (which is called melting) while the change of state that occurs when the water evaporated is from liquid to gas (which is called evaporation).