The ground-state electron configuration of a Cr²⁺ ion is 1s²2s²2p⁶3s²3p⁶3d⁴. Therefore Cr²⁺ is
Answers
The ground-state electron configuration of a Cr2+ ion is 1s2 2s2 2p6 3s2 3p6 3d4. Therefore, Cr2+ is a chromium ion that has lost two electrons from its neutral atom configuration of 1s2 2s2 2p6 3s2 3p6 3d5, resulting in a 3d4 electron configuration.
The ground-state electron configuration of a Cr2+ ion is 1s2 2s2 2p6 3s2 3p6 3d4. Therefore, Cr2+ is an ion with 20 electrons. 1. Chromium (Cr) has an atomic number of 24, meaning it has 24 electrons in its neutral state. 2. Cr2+ indicates that the chromium atom has lost 2 electrons, leaving it with 22 electrons.
3. The given electron configuration (1s2 2s2 2p6 3s2 3p6 3d4) accounts for 20 electrons, meaning there's an error in the configuration. 4. The correct electron configuration for Cr2+ should be 1s2 2s2 2p6 3s2 3p6 3d4 4s2, which accounts for all 22 electrons in the ion.
To know more about electron configuration visit:
https://brainly.com/question/18059044
#SPJ11
Related Questions
PLS HELP ASAP I WILL GIVE THANKS BUT JUST PLS HELP NOW!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Earth
What is the orbit of the Earth?
Is the Sun at the center of the Earth’s orbit?
Describe the motion of the Earth throughout its orbit? Does it move at a constant speed?
Answers
Answer:
The orbit of the earth is a ellipse. Yes. Yes
Explanation:
which balances the equation Mg + O2 → MgO
Answers
Answer:
2Mg+\(O_{2}\)→2MgO
Explanation:
The first step is magnesium + oxygen equals magnesium oxide
The second step is changing that into an equation which looks like Mg+\(O_{2}\)→MgO
The third step is to 2*Mg and 2*O as well as the product has to be 2*Mg,2*O which gets the equation to become balanced as 2Mg+\(O_{2}\)→2MgO
The coefficient 2 balance the given reaction. The balanced equation for the given reaction is given as \(\rm Mg + O_2 \rightarrow 2MgO\).
A balanced equation is a mathematical representation of a chemical reaction that respects the law of mass conservation. It depicts the reactants and products of the reaction, as well as the relative amounts of each species. The unbalanced equation is \(\rm Mg + O_2 \rightarrow MgO\). One magnesium (Mg) atom on the left side and one on the right side, so the magnesium is already balanced. Balance the oxygen (O) atoms. On the left side, there are two oxygen (O) atoms present in the O2 molecule. On the right side, there is only one oxygen (O) atom in the MgO molecule. To balance the oxygen atoms, we need to add a coefficient of 2 in front of MgO: \(\rm Mg + O_2 \rightarrow 2MgO\). The balanced equation is \(\rm Mg + O_2 \rightarrow 2MgO\).
To know more about balanced equation, here:
https://brainly.com/question/12192253
#SPJ4
determine the number of charged particles in nucleus of calcium atom then deduce the number of electrons
NUCLEAR CHARGE (20+)
ATOMIC MASS (40 amu)
given the relative charge of a proton =1+\ m1nucleon=1amu
Answers
Answer:
detail is given below.
Explanation:
The charged particles of nucleus are protons while neutrons are neutral having no charge.
We know that an atom consist of electrons, protons and neutrons. Neutrons and protons are present inside the nucleus while electrons are present out side the nucleus.
Electron has a negative charge and is written as e⁻. The mass of electron is 9.10938356×10⁻³¹ Kg . While mass of proton and neutron is 1.672623×10⁻²⁷Kg and 1.674929×10⁻²⁷ Kg respectively.
Symbol of proton= P⁺
Symbol of neutron= n⁰
The number of electron or number of protons are called atomic number while mass number of an atom is sum of protons and neutrons.
one proton contribute 1 amu to the total weight. There are 20 protons and 20 neutrons in Ca thus its atomic mass is 40 amu.
While the atomic number is 20.
sulfur dioxide has a vapor pressure of 462.7 mm hg at -21.0 c and a vapor pressure of 140.5 mm hg at -44.0 c. what is the molar heat of vaporization of sulfur dioxide? (r = 8.31j/k*mol)
Answers
The molar heat of vaporization of sulfur dioxide is approximately 25,207 J/mol. To calculate the molar heat of vaporization of sulfur dioxide, you can use the Clausius-Clapeyron equation, which is:
ln(P2/P1) = -ΔHvap/R * (1/T2 - 1/T1)
where P1 and P2 are the vapor pressures at temperatures T1 and T2, respectively, ΔHvap is the molar heat of vaporization, and R is the ideal gas constant (8.31 J/K*mol).
First, convert the temperatures from Celsius to Kelvin:
T1 = -21.0°C + 273.15 = 252.15 K
T2 = -44.0°C + 273.15 = 229.15 K
Next, plug the values into the equation:
ln(140.5/462.7) = -ΔHvap/8.31 * (1/229.15 - 1/252.15)
Now, solve for ΔHvap:
ΔHvap = 8.31 * (ln(140.5/462.7) / (1/229.15 - 1/252.15))^-1
ΔHvap ≈ 25,207 J/mol
So, the molar heat of vaporization of sulfur dioxide is approximately 25,207 J/mol.
Learn more about molar heat of vaporization here:
brainly.com/question/29401184
#SPJ11
Would the answer here be b?

Answers
Answer:No the answer is D
Explanation:The reaction between the reactants occurs only when they collide in correct orientation in space. Greater the probability of collisions between the reactants with proper orientation, greater is the rate of reaction. The orientation of molecules affect the probability factor, p. The simple molecules have more ways of proper orientations to collide.
Which two words apply to the substance copper sulphate?
Please give 1 answer.
A.
solid, compound
В.
gas, element
C
solid, mixture
D.
gas, compound
Answers
Answer: A. solid, compound
Explanation:
Because copper sulphate is a compound and it is a blue solid powder
Hope that help :)
Select the correct answer.
Is this equation completely balanced?
2C8H8 + 2502 → 8CO2 + 18H2O
A
No, because the number of carbon, hydrogen & oxygen atoms on both sides of the equation are not equal.
B.
No, because the number of oxygen atoms on both sides of the equation are not equal.
C. Yes, because all of the coefficients are in their lowest whole number ratio.
D.
No, because the number of hydrogen atoms on both sides of the equation are not equal.
E.
Yes, because the number of products is equal to the number of reactants.
Answers
Answer:
A
No, because the number of carbon, hydrogen & oxygen atoms on both sides of the equation are not equal.
Hope this helps!
explain the reason for classifying the periodic table into blocks
Answers
Answer:
The long form of the periodic table divides the elements into four major blocks known as s, p, d, and f.
The period to which a given element belongs can easily be determined from its electron configuration. Based on electron configurations, the periodic table can be divided into blocks denoting which sublevel is in the process of being filled.
Hope it helps.
Given that PbCI2 is insoluble in water, which of the following chemical equations is correct?

Answers
A chemical formula tells you the specific elements included in the compound and the number of atoms of each. The letters in a chemical formula are the symbols for the specific elements. ... The numbers added as a subscript tell you how many atoms of each element are present. If no number is present, there is only one atom.
Are the following chemical equations reversible or irreversible?
2H2O ←→ H3O+ + OH-
HA + H2O ←→ A- + H3O+
HA + H2O → A- + H3O+
MOH → M+ + OH-
Answers
The first two chemical equations are reversible while the other two are irreversible.
What are chemical equations?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
The first chemical equation was put forth by Jean Beguin in 1615.By making use of chemical equations the direction of reaction ,state of reactants and products can be stated. In the chemical equations even the temperature to be maintained and catalyst can be mentioned.
Learn more about chemical equations,here:
https://brainly.com/question/19626681
#SPJ1
Find the number of CO2 molecules present in 20l of CO2 at 0°C and 772mm of Hg.
I wiLL mark you as brainliest and give 50 points.
Answers
Answer:
18
At STP, 22.4 liters of a gas forms one mole that is 6.023×10
23
molecules.
Volume of CO
2
in 1 litre of air =
100
0.03
×1 L
∴ No. of molecules of CO
2
=
22.4
6.023×10
23
×
100
0.03
=8.066×10
18
Explanation:
I guess it's this
\(\\ \sf\longmapsto PV=nRT\)
\(\\ \sf\longmapsto n=\dfrac{PV}{RT}\)
\(\\ \sf\longmapsto n=\dfrac{772(20)}{8.314(273)}\)
\(\\ \sf\longmapsto n=\dfrac{15440}{2269.722}\)
\(\\ \sf\longmapsto n=6.8mol\)
Examine at least three different batteries at home or at a store, each with a different voltage. Make
a chart showing the type of battery (alkaline, NiCd, etc), the voltage, the size, and the use for which each
battery is designed. Why do you think they are different in size and voltage?Examine two items that use more than one battery, such as a flashlight, a 2-way radio, or a battery
operated toy. Look at the arrangement of the batteries in the item. Are they connected in series? For
each item, state the number and type of batteries, the voltage of each battery and the total voltage
produced.
Answers
A battery converts chemical energy to electrical energy.
What is a battery?A battery is a device in which chemical energy is converted to electrical energy. The size of a battery does not necessarily correspond to its voltage.
The images were not shown but a head to head battery connection is said to be in series while a connection of batteries to a common junction is said to be in parallel.
Learn more about series connection:https://brainly.com/question/18713901
Answer:
This is the answer to the first one.
Explanation:
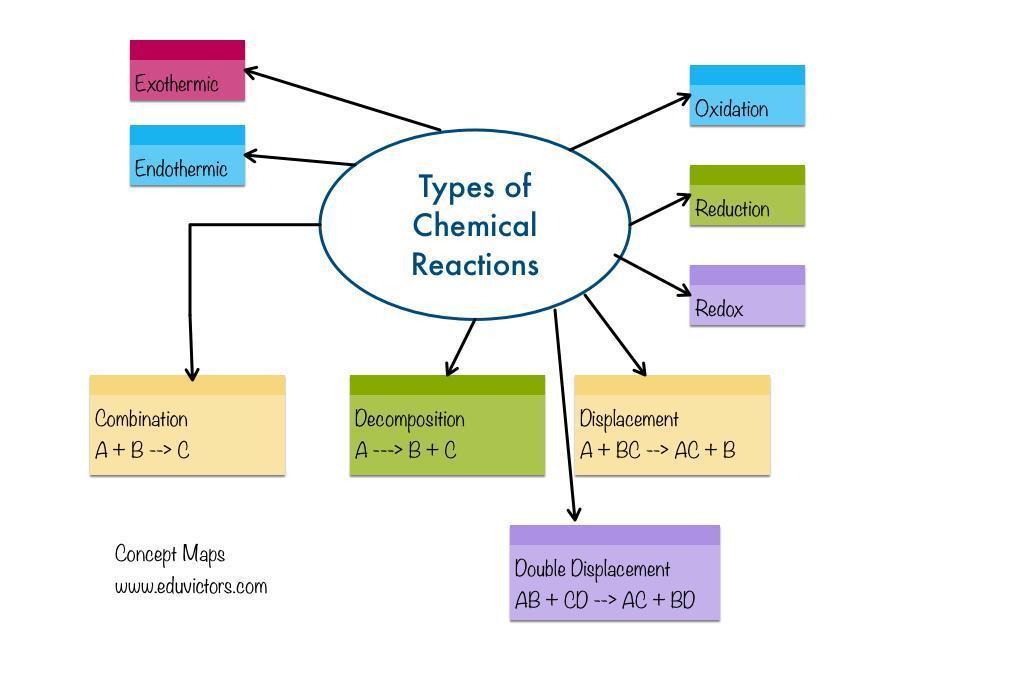
chemical change grade 10 mind map
Answers
Chemical change: A chemical reaction is the change of one chemical substance into another chemical substance. For instance: The rusting of iron, the curdling of milk, the digestion of food, breathing, etc.
What is a chemical reaction?
A chemical reaction results in a chemical change because a new material has entirely different properties from the original substance. In a chemical reaction, atoms rearrange themselves.Reactants are the chemicals that participate in a chemical reaction.Products are the new compounds created as a result of a chemical process. An illustration of a chemical reaction is burning magnesium in the air to produce magnesium oxide.2Mg(s) + O2(g) △→ 2MgO(s)The magnesium ribbon is cleaned with sandpaper before being burned in the air. This cleans the magnesium ribbon's surface of the basic magnesium carbonate protecting coating.Reactant: Materials that participate in a chemical reaction are referred to as reactants. Mg and O2, as an example.A product is a newly created substance that results from a chemical reaction. Example: MgO.A chemical reaction is the change of one chemical substance into another chemical substance.
To learn more about chemical reactions, refer to:
https://brainly.com/question/1222323
#SPJ9

Chemical change is the change chemical substance is transformed into another chemical substance.It is irreversible in nature , for example Reaction of medicine in body , milk to curd etc.
What is the difference between chemical and Physical change?1)Physical change temporary or reversible in nature but chemical change is irreversible in nature
2)In physical change there no new product is formed but in chemical change there formation of new product takes .
3) Physical change is change sin shape ,size or state for example freezing of water , melting of wax , and example of Chemical change are Burning of coal, digestion of food
to learn more about chemical change click here https://brainly.com/question/28089135
#SPJ9
left- and right-handed mirror image molecules are known as
Answers
Left- and right-handed mirror image molecules are known as stereoisomers. Stereoisomers have the same molecular formula and the same connectivity of atoms, but the arrangement of the atoms in space is different. Stereoisomers are formed due to the presence of a chiral center in the molecule
A molecule is said to be chiral if it has a non-superimposable mirror image. Chiral molecules cannot be superimposed on their mirror image. This means that the left- and right-handed mirror images of a chiral molecule are not identical and are not superimposable on each other. Chiral molecules are very important in the field of biology and pharmacology because they interact differently with other chiral molecules in biological systems and can have different biological activities or therapeutic effects.Most biological molecules, such as amino acids, sugars, and DNA, are chiral. Amino acids and sugars are chiral because of the presence of an asymmetric carbon atom in their structures. DNA is chiral because of the helical structure of its double-stranded form. The handedness of chiral molecules can have significant implications for their biological activity, as the interaction between two chiral molecules can depend on their relative handedness.The study of stereoisomers is important in the field of organic chemistry and biochemistry. Understanding the stereochemistry of molecules is essential for understanding their properties and behavior. Stereoisomers can have different physical properties, such as melting point and solubility, and different biological activities, such as receptor binding and enzyme catalysis.
To know more about Chiral molecules visit :
brainly.com/question/29538057
#SPJ11
Which of the following ions could exist in either the high-spin or low-spin state in an octahedral complex?
A. Sc3+
B. Ni2+
C. Mn2+
D. Ti4+
E. Zn2+
Answers
Ni²⁺ is the only ion on the list that can exist as both a high-spin and a low-spin octahedral complex. The correct option is B.
An electrostatic model called the crystal field theory (CFT) assumes that the metal-ligand connection is ionic and results only from electrostatic interactions between the metal ion and the ligand. When dealing with anions, ligands are viewed as point charges, and when dealing with neutral molecules, as dipoles.
The crystal field splitting theory predicts that some transition metal ions can exist as either high-spin or low-spin octahedral complexes, depending on the magnitude of the crystal field splitting parameter (Δ) relative to the pairing energy (P).
Of the ions listed, the only one that could exist as either a high-spin or a low-spin octahedral complex is Ni²⁺ (B).
Mn²⁺ (A) is a d⁵ ion and will always form a high-spin octahedral complex due to its large number of unpaired electrons.
Sc³⁺ (C) is a d⁰ ion and does not form octahedral complexes with ligands.
Cu²⁺ (D) is a d⁹ ion and typically forms a low-spin octahedral complex due to the stability of the half-filled d⁹ configuration.
Zn²⁺ (E) is a d¹⁰ ion and does not have any unpaired electrons to undergo spin pairing, so it will always form a low-spin octahedral complex.
Therefore, the correct answer is B) Ni²⁺.
To know more about crystal field theory here
brainly.com/question/23840749
#SPJ4
Which of the following is an extensive property of a sample of aluminum?
Answers
Answer:
Entropy,mass and volume.... I think
Mass is the extensive property of a sample of aluminum. Therefore, option (A) is correct.
What is the extensive property of matter?The extensive property can be described as a physical property of matter that changes with a change in the size and shape of the matter. Therefore, the extensive property of any substance varies directly with the mass.
Extensive properties are the value of the property of the system that must be equal to the sum of the values of different parts of the system. These properties depend on the amount of matter contained in the system.
Examples of Extensive properties are temperature, pressure, density, boiling point, etc. The ratio of the two extensive properties is an intensive property such as density.
The mass of a sample of aluminum is an example of extensive property as it depends on the amount of aluminum in the given sample. This property of the sample is proportional to the size of the system.
Learn more about the extensive property, here:
https://brainly.com/question/13733851
#SPJ6
Your question is incomplete, most probably the complete question was,
Which of the following is an extensive property of a sample of aluminum?
A) Mass
B) Temperature
C) Pressure
D) Density
The photograph shows one way individuals work together in groups. What
kind of work is shown in the photograph?
Answers
A gas has an initial volume of 15 L. If the temperature increases from 330 K to 450 K, what is the new volume.
Answers
Answer:
20.(45)L or about 20.4545L
Explanation:
PV = nRT
Where:
P - pressure
V - volume
n - number of particle moles
R - a constant
T - temperature in K
We can assume the P and n (and definitely R) stay the same, so we infer that
\(V_1 = \frac{nRT_1}{P} = 15L\\V_2 = \frac{nRT_2}{P}\\V_1 / V_2 = \frac{nRT_1}{P} / \frac{nRT_2}{P} = \frac{T_1}{T_2}\\\\15L / V_2 = \frac{330K}{450K} = \frac{11}{15}\\\\V_2 = 15L \cdot \frac{15}{11} = 20.(45)L\)
What does the dropping liquid create
Answers
What is Decomposition Reaction
Answers
Answer:
Explanation:
Decomposition reaction, also known as analysis or dissociation, is a type of chemical reaction in which a compound breaks down into simpler substances or elements. In this reaction, a single reactant undergoes a chemical change and produces two or more products.
The decomposition reaction can be represented by the general equation:
AB → A + B
Where AB is the reactant, and A and B are the products. The reactant AB is usually a compound, and it breaks down into its constituent elements or simpler compounds.
There are different types of decomposition reactions, including:
Thermal decomposition: It occurs when a compound is heated, resulting in its decomposition into simpler substances. For example, the thermal decomposition of calcium carbonate (CaCO3) produces calcium oxide (CaO) and carbon dioxide (CO2):
CaCO3 → CaO + CO2
Electrolytic decomposition: It takes place when an electric current is passed through an electrolyte, causing it to break down into its component ions. For instance, the electrolysis of water (H2O) leads to the decomposition into hydrogen gas (H2) and oxygen gas (O2):
2H2O → 2H2 + O2
Photochemical decomposition: It occurs when a compound undergoes decomposition due to exposure to light energy. Chlorine gas (Cl2) can decompose into chlorine atoms (Cl) under the influence of light:
Cl2 → 2Cl
These are just a few examples of decomposition reactions. They are important in various chemical processes and are used in industries, laboratory experiments, and natural phenomena. By understanding and controlling decomposition reactions, scientists can gain insights into the behavior of different compounds and develop practical applications in fields such as chemistry, materials science, and environmental science.
Answer:
Explanation:
reaction in which a compound breaks down into simpler substances or elements
A 118.0 g sample of a compound contains 72.0 g of C. 18.0 g of H, and 28.0 g of N.
Which of the following is the empirical formula of the compound?
Answers
The empirical formula of the compound with 72.0 g of C. 18.0 g of H, and 28.0 g of N is C₃H₉N.
How to calculate empirical formula?Empirical formula of a compound indicates the ratios of the various elements present in a compound, without regard to the actual numbers.
The empirical formula of a compound can be calculated as follows:
C = 72.0g H = 18.0g N = 28.0gFirst, we divide by the atomic mass of each element as follows:
C = 72.0g ÷ 12 = 6molH = 18.0g ÷ 1 = 18 molN = 28.0g ÷ 14 = 2molNext, we divide by the smallest mole as follows:
C = 6mol ÷ 2 = 3
H = 18 mol ÷ 2 = 9
N = 2mol ÷ 2 = 1
The empirical ratio is 3:9:1, hence, it can be said that the empirical formula is as follows: C₃H₉N.
Learn more about empirical formula at: https://brainly.com/question/14044066
#SPJ1
Which of the following is an example of acceleration?
Оа
Ob
Ос
Od
A car moves at a constant speed.
A car is at rest.
A car covers 10 miles of distance every 10 minutes.
A car speeds up from 0 km/hr to 100 km/hr in 6 seconds.
Answers
Answer:
A car speeds up from 0 km/hr to 100 km/hr in 6 seconds.
Explanation:
2.5 silly is equal to how many kilosilly?
Answers
Answer:
2.5 silly = 0.00025 kilosilly
Explanation:
To convert: \(2.5\) silly to kilosilly
Solution:
Relation between \(2.5\) silly and kilosilly is given as follows:
1 silly = \(\frac{1}{1000}\) kilosilly
Now use this formula to convert \(2.5\) silly to kilosilly.
\(2.5\) silly\(=\) \(\frac{2.5}{1000}\) kilosilly
Here, \(\frac{2.5}{1000} =0.00025\)
So,
2.5 silly = 0.00025 kilosilly
If a pea plant is hybrid for height, this means that
a) It has one dominant and one recessive allele
b) it has two of the same allele
PLSSSS HELP ASAP!!
Answers
Answer:
A
Explanation:
By nature, a hybrid has one dominante allele and one recessive allele
True or False: The relative concentrations of ATP and ADP control the cellular rates of the citric acid cycle
Answers
True. The relative concentrations of ATP and ADP play a critical role in controlling the cellular rates of the citric acid cycle, also known as the Krebs cycle or the tricarboxylic acid (TCA) cycle.
The citric acid cycle is a series of reactions that occur in the mitochondria and generates energy by oxidizing acetyl-CoA. This energy is stored in the form of ATP, which is used to power various cellular processes.
When the cellular ATP levels are high, the rate of the citric acid cycle slows down because the cell has sufficient energy and does not need to produce more. On the other hand, when the cellular ATP levels are low, the rate of the citric acid cycle increases to produce more ATP.
Learn more about citric acid cycle here:
https://brainly.com/question/29857075
#SPJ11
What is science? Write a definition for the word
Answers
Answer:
make me brainliest pls :)
Explanation:
Science is both a body of knowledge and a process. In school, science may sometimes seem like a collection of isolated and static facts listed in a textbook, but that's only a small part of the story. Just as importantly, science is also a process of discovery that allows us to link isolated facts into coherent and comprehensive understandings of the natural world.
Science is exciting. Science is a way of discovering what's in the universe and how those things work today, how they worked in the past, and how they are likely to work in the future. Scientists are motivated by the thrill of seeing or figuring out something that no one has before.
Science is useful. The knowledge generated by science is powerful and reliable. It can be used to develop new technologies, treat diseases, and deal with many other sorts of problems.
Science is ongoing. Science is continually refining and expanding our knowledge of the universe, and as it does, it leads to new questions for future investigation. Science will never be "finished."
Science is a global human endeavor. People all over the world participate in the process of science. And you can too!
The density of steel is about 8.0g/cm³ and the density of water is 1.0 g/cm³ . If the mass of steel used for the ship is 50,000 kg, what would the volume need to be for the ship to float on water?
Answers
In order for the ship to float, the volume of the ship must be equal to or greater than 50,000,000 cm³.
What would be the volume of the ship in order for it to float on water?According to the principle of floatation, a body will float in a liquid if it displaces a weight of liquid equal to its own weight.
Therefore, for the ship to float in water, it must displace a weight of water at least equal or greater than its own weight.
Considering the data given:
The density of steel = 8.0g/cm³
the density of water is 1.0 g/cm³
the mass of steel used for the ship = 50,000 kg
Based on the principle of floatation, mass of water to be displaced is equal to the mass of the ship.
Mass of water to be displaced = 50000 kg
Mass of water to be displaced in g = 50,000,000 g
Volume of 50000000 g of water = 50,000,000 g * 1.0 g/cm³
Volume of 50000000 g of water = 50,000,000 cm³
Therefore, volume of the ship must be equal to or greater than 50,000,000 cm³ for the ship to float.
Learn more about principle of floatation at: https://brainly.com/question/17032479
#SPJ1
Put these stars in order from least luminous to most luminous: red giant K star, supergiant F star, white dwarf A star.
Question 6 options:
White dwarf A, supergiant F and red giant K
White dwarf A, red giant K, and supergiant F
Supergiant F, white dwarf A, and red giant K
Red gaint K, supergiant F, and white dward A

Answers
Answer B
From least luminous to most luminous:
White dwarf A star - White dwarfs are small, dense, and dim stars that have exhausted their nuclear fuel and have cooled down. They emit very little light, and their luminosity is much lower than that of most other types of stars.
Red giant K star - Red giants are large and relatively cool stars that have exhausted the hydrogen fuel in their cores and have expanded to several times their original size. They are brighter than main-sequence stars like our Sun but less bright than supergiants.
Supergiant F star - Supergiants are massive and luminous stars that are in the last stages of their lives. They are much larger and brighter than most other types of stars, including red giants, and have a high luminosity. F-type supergiants are particularly bright and have a high surface temperature.
which of these elements is least reactive?
Lithium
Beryllium
Potassium
Calcium
Answers
Answer:
Beryllium because on the periodic table the metal reactivity is decreased
Be sure to answer all parts. Compounds a and b are isomers having molecular formula c5h12. Heating a with cl2 gives a single product of monohalogenation, whereas heating b under the same conditions forms three constitutional isomers. What are the structures of a and b?.
Answers
Neo-pentane represents the Compound A while compound B is n-pentane.
After careful consideration we can say that compounds A and B are alkanes and also isomers of pentane. In chemistry, Isomers are defined as compounds having same empirical molecular formula but different structural formulas due to varying arrangement of atoms.
Now, as per the question statement, compound A gives a single monochlorination product upon heating with the molecule of chlorine i.e. Cl2 showing that the molecule is extremely symmetric. This molecule must be neo-pentane. Refer to image 1.
Similarly, Compound B forms 3 constitutional isomers after undergoing monochlorination. This compound must be n-pentane since three are 3 different types of carbon atoms in the structure. Refer to image 2.
If you need to learn more about neo-pentane click here:
https://brainly.com/question/20815247
#SPJ4


Neopentane makes up component A, while n-pentane makes up compound B.
First and foremost, it is important to understand that compounds A and B are isomers and alkanes of pentane. Compounds with distinct structural formulas but the same molecular formula are known as isomers.
When heated with Cl2, compound A now produces a single monochlorination product, demonstrating the molecule's high degree of symmetry. Neopentane must be this chemical (image 1).
Upon monochlorination, compound B divides into three constitutional isomers.
A halogen atom is replaced with another substance in a process known as halogenation, where the halogen atom eventually becomes a component of the new substance or compound. In general, one or more halogens are typically added to the chemical during the halogenation reaction.
Learn more about halogens:
https://brainly.com/question/14191541
#SPJ4