The graph below shows how the temperature and volume of a gas vary when the number of moles and the pressure of the gas are held constant. How can the volume of the gas be increased if the pressure is constant?

Answers
Answer:
Option C. By increasing the temperature
Explanation:
From the graphical illustration above, we see clearly that the volume and temperature of the gas are directly proportional. This implies that as the temperature increases, the volume will also increase and as the temperature decreases, the volume will also decrease. This can further be explained by using the ideal gas equation as shown below:
PV = nRT
P is the pressure.
V is the volume.
n is the number of mole.
R is the gas constant.
T is the temperature.
PV = nRT
Divide both side by P
V = nRT/P
Since n and P are constant, the equation above becomes:
V & T
V = KT
K is the constant.
The above equation i.e V = KT implies that:
As T increases, V will also increase and as T decreases, V will also decrease.
Considering the question given above,
The volume of the gas can be increased if the temperature is increased.
Related Questions
Zelda noticed a puddle outside her front door. She saw that the puddle got smaller every day, until the 3rd day when it was completely gone. The next week, she noticed the puddle again. This time the puddle was gone the next day. Since the sun was out the second week but not the first week, Zelda hypothesized that the heat from the sun was the reason for the water evaporating at a faster rate. If she were to set up two containers with equal amounts of water, what would be the best way for Zeldato test her hypothesis\
Answers
Answer: Zelda should place one container of water in sunlight (by a window or outdoors) and the other container in a dark room (closet) away from the sun.
Explanation: This would allow Zelda to test two different settings (sun and no sun) so she can test her hypothesis.
chegg which one of the following is not a possible product when a crossed aldol addition reaction is carried out with ethanal and butanal as reactants?
Answers
The correct answer is option 4: 2,3-Dimethyloxan-4-one. In a crossed aldol addition reaction, two different aldehydes or ketones are used as reactants to form a β-hydroxy aldehyde or ketone.
The reaction involves the nucleophilic addition of an enolate ion to the carbonyl group of the other reactant, followed by dehydration.
When considering the reaction between ethanal (CH3CHO) and butanal (CH3(CH2)2CHO), there are several possible products that can be formed. Let's evaluate the options:
3-Hydroxybutanal (CH3(CH2)2CH(OH)CHO): This product can be formed by the nucleophilic addition of the enolate of ethanal to the carbonyl group of butanal. It is a valid product.
4-Hydroxybutanal (CH3(CH2)2CHOHCHO): This product can also be formed by the nucleophilic addition of the enolate of butanal to the carbonyl group of ethanal. It is a valid product.
2-Hydroxy-2-methylbutanal (CH3(CH2)2C(OH)(CH3)CHO): This product can be formed by the nucleophilic addition of the enolate of ethanal to the carbonyl group of butanal, followed by dehydration and methyl migration. It is a valid product.
2,3-Dimethyloxan-4-one: This compound does not correspond to a possible product resulting from the crossed aldol addition between ethanal and butanal. It does not involve the formation of a β-hydroxy aldehyde or ketone. Therefore, option 4 is not a possible product in this reaction.
Therefore, the correct answer is option 4: 2,3-Dimethyloxan-4-one.
learn more about Dimethyloxan here
https://brainly.com/question/4279223
#SPJ11
Ethene is exposed to h2 in a solution containing a chunk of solid palladium. if it reacts successfully, the original alkene will:________
Answers
If ethene and hydrogen reacts successfully in a solution containing a chunk of solid palladium, the original alkene will become unsaturated.
What is hydrogenation?The term hydrogenation is used in chemistry to describe that addition of hydrogen to an unsaturated compound. Recall that ethene is an un saturated compound (alkene) and one of the reactions that an unsaturated compound can undergo is the addition of atoms or group across the double bond to yield adducts.
In this case, the adduct is a compound that is saturated ad will not have the same properties as ethene because the carbon carbon double bond that was characteristic of ethene has been replaced by a carbon carbon single bond.
Thus, if ethene and hydrogen reacts successfully in a solution containing a chunk of solid palladium, the original alkene will become unsaturated.
Learn more about alkene:https://brainly.com/question/13910028
#SPJ1
A 634.5 g sample of helium absorbs 125.7 calories of heat. The specific heat capacity of helium is 1.241 cal/(g·°C). By how much did the temperature of this sample change, in degrees Celsius?
Answers
Answer:
\(\Delta T=0.160\°C\)
Explanation:
Hello there!
In this case, according to the following equation for the calculation of heat in this calorimetry problem:
\(Q=mC\Delta T\)
It is possible for us to calculate to calculate the change in temperature for this process by solving for DT in the aforementioned equation:
\(\Delta T=\frac{Q}{mC}\\\\ \Delta T=\frac{125.7cal}{634.5g*1.241 cal/(g\°C)} \\\\ \Delta T=0.160\°C\)
Best regards!
why water has more than one atom?
Answers
Answer:
In general, electrons can be shared between atoms (a molecular bond) or electrons can be completely removed from one atom and given to another (an ionic bond). ... Water is a molecule because it contains molecular bonds. Water is also a compound because it is made from more than one kind of element (oxygen and hydrogen).
Explanation:
The mass number is used to calculate the number of___________ in one atom of an element. In order to calculate the number of neutrons you must subtract the ______________________ from the________
Answers
Answer:
Neutrons, Atomic Number, Atomic Mass
Explanation:
The Atomic mass is used to calculate the number of Neutrons in an atom.
Every atom is composed of Protons and Neutrons forming a tight compact nucleus orbited by electrons. The Atomic number of an element tells how many Protons the nucleus has. This is important because it determines how many electrons the atom has and consequently, its chemical properties. The Atomic mass (rounded to the nearest whole number) is the sum of the Protons and Neutrons in the elements nucleus, since their masses are nearly identical (Neutrons have one electron worth more mass than Protons). You subtract an element's Atomic number from its Atomic mass and you get the number of neutrons the element has in the nuclei of its atoms.
question at position 1 sand is mostly composed of small particles of silicon dioxide. to what materials class would you classify sand?
Answers
Sand is absolutely composed of small particles of silicon dioxide. Sand can be classified as ceramics.
The silicon dioxide is a compound. It is an oxide of Silicon. The oxide are formed by the reaction of an element with the oxygen. In silicon dioxide, the silicon reacts with two oxygen molecule to form silicon dioxide. So, the two elements present in the silicon dioxide would be silicon and oxygen.
Ceramics can be defined as inorganic non-metallic materials which harden at high temperatures. The atomic structure of ceramics can be crystalline, non-crystalline, or semi-crystalline. However, most ceramics have a crystalline atomic structure.
Learn more about ceramics:
brainly.com/question/29455196
#SPJ4
In a chemical change
Answers
Answer:
Chemical changes occur when a substance combines with another to form a new substance, called chemical synthesis or, alternatively, chemical decomposition into two or more different substances. These processes are called chemical reactions and, in general, are not reversible except by further chemical reactions.
Explanation:
What BEST describes the Kleercut campaign?
Protesting rarely produces positive results.
Greenpeace’s tactics are similar to those of ecoterrorists.
Nonprofits have no role in determining forestry practices.
Nonprofits and corporations can work together to make sound policy.
Answers
Tactics are similar to those of ecoterrorists. This best describes the campaign. Therefore, the correct option is option B.
Natural Resources Defence Council, and others once engaged in a campaign against Kimberly-Clark known as campaigns. From 2004 to 2009, it took place. The largest producer of tissue products in the world, Kimberly-Clark is well known for its Kleenex brand.
Every year, the company buys from logging companies more than three million kilogrammes (3.4 million tonnes) of fibre. According to the marketing campaign, this fibre is made with old growth forest wood pulp. Tactics are similar to those of eco. This best describes the Kleercut campaign.
Therefore, the correct option is option B.
To know more about campaign, here:
https://brainly.com/question/30293626
#SPJ1
A. compound has the formula X3Y. If there are 15 X atoms, what is the coefficient in front of the compound
Answers
If there are 15 X atoms, the coefficient in front of the compound X3Y will be 5.
Different quantity of atoms of specific elements exist in a compound. According to this question, the compound with chemical formula: X3Y was given. This means that the compound consists of elements X and Y. Coefficients, which are numbers placed in front of an element, are used to balance the number of atoms contained in that element. If there are 15 atoms of X in the compound X3Y, then a coefficient of 5 will be used to complete the number of atoms in the compound as follows: 5X3Y.Learn more at: https://brainly.com/question/17274608?referrer=searchResults
What is the frequency of a photon with an energy of 3.38 x 10-19 J?
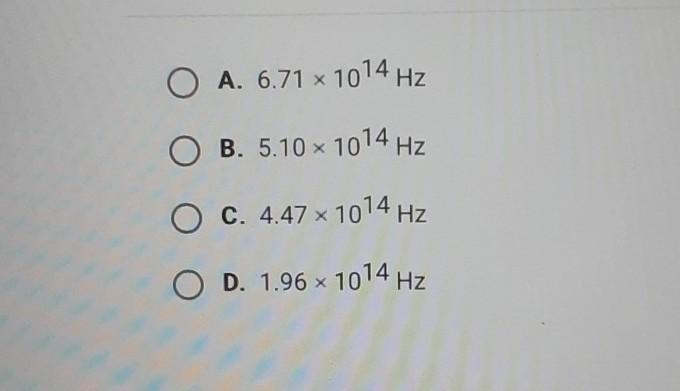
Answers
\(\huge\boxed{5.1x\(10^{14}\)Hz}\)
_____________________________________DATA:E = \(3.38 x 10^{-19}\)
h = \(6.625x10^{-34}\)
f = ?
_____________________________________SOLUTION:Energy of Photon is given by,
E = hf
Rearrange the equation,
f = \(\frac{E}{h}\)
where,
E = energy
h = planck's constant
f = frequency
------------------------------------------------------------------------------------------------------------
Substitute the variable in the equation,
f = \(\frac{3.38x10^{-19}}{6.62x10^{-34}}\)
Simplify the equation,
f = 5.10 x 10^14 Hz
_____________________________________Best Regards,'Borz'
Answer:
b. 5.10 x 10^14 Hz
Explanation:
3. Which best explains the flow of energy in an internal combustion engine?
O
A. Work is converted into energy stored in chemical bonds.
B. The expansion of gases is used to do work.
C. Heat is converted into work to power the engine.
D. An increase in pressure is converted into heat and work.
Check Answer
Answers
An increase in pressure is converted into heat and work, explains the flow of energy in an internal combustion engine. Gasoline is ignited and consumed within an internal combustion engine (ICE) by the engine itself.
The engine then partially transforms the energy from combustion into work. The engine is composed of a fixed cylinder and a rotating piston. There are three main types of internal combustion engines in use today: the spark ignition engine, which is mainly used in automobiles; the diesel engine, which is used in heavy machinery and industrial systems and has an advantage over the more compact and lighter ones due to improvements in cycle efficiency.
To learn more about engine, click here.
https://brainly.com/question/19117846
#SPJ1
How do chloroplasts contribute to the function of the cell?
They convert energy from the sun into glucose.
They provide structure to the cell.
They enclose the cell and separate it from other things in its environment.
They convert glucose into usable energy.
Answers
Answer:
I think the answer is "They enclose the cell and separate it from other things in its environment".
Explanation:
I am terribily sorry if this is the wrong answer, I was trying to help :\
Chloroplasts contribute to the function of the cell because they CONVERT energy from the sun into glucose.
Chloroplasts are organelles found in plants and some algae that act to harvest the energy from the sun in order to produce ATP and subsequently simple carbohydrates (glucose) by a process known as photosynthesis.
Photosynthesis has two types of reactions: light-dependent reactions and light-independent reactions.
The light-dependent reactions that produce ATP occur in the thylakoid membrane of the chloroplasts.
The light-independent reactions that produce simple carbohydrates occur in the stroma of the chloroplast.
In conclusion, chloroplasts contribute to the function of the cell because they CONVERT energy from the sun into glucose.
Learn more in:
The strategy of using an alkene intermediate in a two-step process to convert one functional group into another called a functional group interconversion) can apply to a wide variety of transformations. Identify the reagents you would use to accomplish the following functional group interconversion
Answers
We can see conversion of an alcohol to an alkane.
Reagents needed:
1. Sodium or Potassium Hydride.
2. Hydrogen Gas.
What is a functional group?
A functional group is a group of atoms or molecules within a larger molecule that is responsible for the molecule's chemical and physical properties. Functional groups have specific chemical characteristics, such as polarity, reactivity, and solubility, that give the molecule its unique properties.
What is functional group interconversion?
Functional group interconversion is a type of chemical reaction in which one functional group is converted into another functional group. This type of reaction is used to modify the properties of a molecule, such as its reactivity, solubility, and stability. Examples of functional group interconversion include the conversion of an alcohol to an aldehyde, an aldehyde to a ketone, and an ester to an amide.
To know more about Functional group,
https://brainly.com/question/10058230
#SPJ4
5.2 kg of argon fills an insulated, rigid container which has a volume of 0.8 . if the temperature within the container is 83 , what is the pressure of the argon in kpa?
Answers
We can solve the problem using the Ideal Gas Law which states that:
PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant and T is the temperature.
Rearranging this equation, we get:
P = nRT/V.
We have to find the pressure of argon in kPa given that it fills an insulated, rigid container with a volume of 0.8 m3 and the temperature within the container is 83°C. The number of moles can be calculated as:
n = mass/molar mass = 5.2 kg/39.948 g/mol = 130.22 moles.
The gas constant R is equal to 8.314 J/(mol K).
The temperature has to be in Kelvin, which is equal to= 83°C + 273.15 = 356.15 K.
Therefore, the pressure can be calculated.
The pressure of the argon in kPa is 3696.98
To know more about Gas Law visit:
brainly.com/question/30458409
#SPJ11
True or false ??
compounds and mixtures are made
up of two or more elements that can be physically separated.
Answers
Which of the following examples involves an exothermic change.
a. ice melting on a warm day
b. water boiling in a tea kettle
c. gaseous water particles coming together to form fog
d. air in a bicycle tire gaining pressure after a long ride
Answers
Which of the following examples involves an exothermic change.
Answer : C
Explanation : Gaseous water particles coming together to form fog
Hope this helps you!
Gaseous water particles coming together to form fog is an exothermic change due to release of thermal energy.
What is exothermic change?
An exothermic reaction is defined as a reaction that releases heat energy to the surrounding environment. It include any combustion process, rusting of iron, and freezing of water.
In conclusion, we can say that Gaseous water particles coming together to form fog is an exothermic change due to release of thermal energy.
Learn more about exothermic here: https://brainly.com/question/2924714
What about 50 g of water?
I need help what this
Answers
Answer:
3.38 Tablespoons
10.14 Teaspoons
0.21 U.S. Cups
0.18 Imperial Cups
0.20 Metric Cups
50.00 Milliliters
Explanation:
Definition: The time in an age-structure diagram where the population is not yet reproducing.
Answers
Answer:
Explanation:An age-structure diagram provides a snapshot of the current population and can represent information about the past and give potential clues about future problems. When you are interpreting age-structure diagrams, it is important to compare the width of the base to the rest of the population
The instructor reveals that the weak acid is hypochlorous acid, HOCl. The known Ka for HOCl is 4.0×10-8. What is the percent error in the experiment?
Answers
The acid is weak. In this acid, the chlorine atom is in the +3 oxidation state. The pure material decomposes into hypochlorous acid (Cl oxidation state +1) and chloric acid (Cl oxidation state +5) and is unstable.
Is HOCl an effective acid?As chlorine dissolves in water, a weak acid called hypochlorous acid (ClOH, HClO, HOCl, or ClHO) is produced. Hypochlorous acid then partially dissociates to produce hypochlorite, ClO.
Does HOCl hurt skin?HOCl is extremely gentle on your skin and harmless, handling this demanding work. Its production by your body's immune system naturally results in its softness. It works well for delicate skin, says Dr.
To know more about acid visit:-
brainly.com/question/14072179
#SPJ1
suppose a rhodium atom in the oxidation state formed a complex with one iodide anion and five ammonia molecules. write the chemical formula of this complex.
Answers
The chemical formula of this complex would be [Rh(NH3)5I].
To write the chemical formula for the complex formed by a rhodium atom in the oxidation state, one iodide anion, and five ammonia molecules, follow these steps:
1. Determine the oxidation state of rhodium (Rh). Since it's not provided, we will represent it with "x."
2. Write the chemical symbols and charges for each component of the complex: Rh (x), iodide (I⁻), and ammonia (NH₃).
3. Combine the components to form the complex. Rhodium will be in the center, and the ligands (iodide and ammonia molecules) will surround it. Remember that there's one iodide anion and five ammonia molecules.
The chemical formula for this complex is [Rh(NH₃)₅I]^(x-1), where "x" is the oxidation state of the rhodium atom.
learn more about chemical here
https://brainly.com/question/28945073
#SPJ11
0.92 lbm of water fills a container whose volume is 1.92 ft3. the pressure in the container is 100 psia. calculate the total internal energy and enthalpy in the container. use data from the steam tables. the total internal energy in the container is btu. the enthalpy in the container is btu.
Answers
The total internal energy in the container is 329.77 Btu and the enthalpy in the container is 385.14 Btu.
Using the steam tables, we can determine the specific volume of water at the given pressure and temperature. The specific volume of water is 0.01658 \(ft^3/lbm\).
The mass of water in the container is 0.92 lbm, so the total volume of the water is:
V = m/v = 0.92 lbm / 0.01658 \(ft^3/lbm\) = \(55.539 ft^3\)
Assuming the water is at saturation, we can find the total internal energy and enthalpy by using the values in the steam tables for saturated water at 100 psia.
From the steam tables, the total internal energy of saturated water at 100 psia is 358.05 Btu/lbm, so the total internal energy in the container is:
U = m * u = 0.92 lbm * 358.05 Btu/lbm = 329.77 Btu
From the steam tables, the enthalpy of saturated water at 100 psia is 419.02 Btu/lbm, so the enthalpy in the container is:
H = m * h = 0.92 lbm * 419.02 Btu/lbm = 385.14 Btu
Therefore, the total internal energy in the container is 329.77 Btu and the enthalpy in the container is 385.14 Btu.
Learn more about enthalpy :
https://brainly.com/question/13996238
#SPJ4
A silver-colored metal is placed in a blue solution. After a few minutes, a red coating forms
on the metal and the solution turns clear. Which best describes the products of this
reaction?
Answers
The reaction when silver metal is coated with red color after the reaction is known as displacement reaction.
The silver colored metal is coated with red color after reaction so the coating is a single element which is displaced by the silver colored reactant and the silver colored metal will form a compound because it displaces the red colored metal. So the products are a single metal and a compound and the reaction is displacement reaction.
Thus, the reaction describes a displacement reaction.
To learn more about metal check the link below:
https://brainly.com/question/25597694
#SPJ4
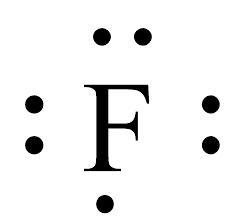
Draw the correct Lewis dot structure from the given shorthand notation below: PLS HELP

Answers
The Lewis structure of the element have been shown in the image attached.
Lewis dot structure of an element:The valence electrons of an atom or molecule are depicted in a simplified manner by the Lewis structure, commonly referred to as the Lewis dot structure or electron dot structure. Gilbert N. Lewis, an American scientist, created it.
The valence electrons of an atom are shown in a Lewis structure as dots surrounding the element's symbol. These dots' placement reveals details about the connectivity and atom-atom bonding in a molecule.
Learn more about Lewis structure:https://brainly.com/question/29756546
#SPJ1
1. explain how u would test the presence of oxygen and hydrogen gases
2. explain how u can get the most accurate reading in titration
3. why is scientific method important in chemistry? state your opinion
4. you are given a bucket of ice cubes, a little sugar, a measuring cylinder and a stopwatch.Plan a experiment to determine whetther sugar increases the melting rate of ice
Answers
sorry
i don’t know but maybe next time
Why would it not be pratical to use water for a barometer on a regular basis? what other liquid might be a good option?.
Answers
Therefore, if you utilise water, you must have a barometer that is 13.534 times the length of a mercury barometer, which may be longer than 11 metres.
Additionally, mercury can reach the same temperature of the atmosphere more quickly than water since it has a lower specific heat than water and is a better heat conductor.
What is Barometer?
Barometer, a tool for calculating atmospheric pressure. A barometer can also be used to determine height because air pressure varies with elevation above or below sea level. Mercury and aneroid barometers are the two primary varieties.
A mercury column whose height can be precisely measured is balanced by atmospheric pressure in a mercury barometer. Mercury barometers are frequently adjusted for the local gravity value and the surrounding temperature in order to boost their accuracy.
Learn more about Barometer from given link
https://brainly.com/question/24939205
#SPJ4
3. Study the information in the table below and answer the questions that follow The letters do not represent the actual symbols of the elements. Element Electronic configuration X Y Z 2.7 2.87 2.8.8.7 Boiling point -188 C -35 C 59°C (a) What is the general name given to the group in which the elements X, Y and Z belong? Select two which are gases (c) Explain why Z has the highest boiling point (d) Write an equation for the reaction of element Z with iron metal (e) Element Y was dissolved in water and a piece of blue litmus paper was put into the resulting solution. State and explain the observation that was made on the litmus paper & B.C.E. F. and G. Elements in group X have a valmey
Answers
(a) The halogen group includes the elements X, Y, and Z. Z is a solid, X and Y are gases. (c) Because Z contains the most electrons in its outermost shell, it has the highest boiling point. It has the highest intermolecular interactions, which makes it more difficult to separate and necessitates a greater temperature to attain its boiling point. (
d) Fe + Z FeZ is the equation for the reaction between element Z and iron metal. (e) If the fluid is acidic, the litmus paper will become red.
This is so because element Y is a halogen, which when dissolved in water may produce hydrohalic acids. These acids are potent enough to transform blue litmus paper to red.
Learn more about Electronic configuration at:
https://brainly.com/question/31812229
#SPJ1
Which of the following is not an ionic compound

Answers
Answer:
bR2 since it is nonpolar
perform a retrosynthetic analysis of the alcohol. the compound consists of a carbon bonded to a benzene ring, an ethyl group, a propyl group and a hydroxy group. the alcohol is , so it is formed by reaction of the grignard reagent with which structure cannot be a reagent or starting material in the synthesis?
Answers
Answer:
yes it is correct 10000000
Please help :)
It’s a science thing for 7th grade

Answers
Answer: The first question is chemical The second one is Physical
Explanation: