the gas, neon, is found in dry air at sea level at a concentration of 1.82×10-3 percent by volume. what is the concentration of ne expressed in ppm?
Answers
Given that the gas, neon, is found in dry air at sea level at a concentration of 1.82×10-3 percent by volume. We have to determine the concentration of ne expressed in ppm.
To determine the concentration of ne expressed in ppm, we use the formula:ppm (parts per million) = (parts / total) * 10⁶Here, parts = volume of ne in dry air at sea level = 1.82×10-3 percent by volume Total = Total volume of dry air at sea levelThe volume of air at sea level is 1.25 × 104 m³.
Let's substitute the values in the formula: ppm = (1.82×10-3 / 100) * 10⁶ppm = 18.2Neon is present in dry air at sea level at a concentration of 18.2 ppm (parts per million).Thus, the concentration of ne expressed in ppm is 18.2 ppm.
To know more about gas visit :
https://brainly.com/question/14812509
#SPJ11
Related Questions
Use standard enthalpies of formation to calculate ΔH∘rxn for the following reaction. Mg(OH)2(s)→MgO(s)+H2O(g)
Answers
The value of ΔH∘rxn is -804.3 kJ/mol, under the condition that the given reaction is Mg(OH)₂(s)→hMgO(s)+ H₂O.
The given standard enthalpy of formation of Mg(OH)2 is +37.1 kJ/mol and that of MgO is -601.6 kJ/mol. Utilizing these values, we can evaluate the standard enthalpy change for the reaction
ΔH∘rxn = ΣnΔH∘f(products) - ΣmΔH∘f(reactants)
Here
ΔH∘f = standard enthalpy of formation for each species
n and m = specific stoichiometric coefficients concerning the products and reactants.
For this given reaction,
Mg(OH)₂(s) → MgO(s) + H₂O(g)
So n = 1 and m = 1.
Staging the values
ΔH∘rxn = [ΔH∘f(MgO) + ΔH∘f(H₂O)] - ΔH∘f(Mg(OH)₂)
ΔH∘rxn = [-601.6 kJ/mol + (-241.8 kJ/mol)] - (+37.1 kJ/mol)
ΔH∘rxn = -804.3 kJ/mol
To learn more about enthalpy
https://brainly.com/question/16985375
#SPJ4
Which will most likely happen to her soup?
Carlotta adds too much salt to her soup. She recalls that
evaporation can be used to separate salt from salt water,
so she plans to leave the soup on the stove on low heat
until the soup is less salty.
O The soup will become saltier because evaporation
removes the water and leaves the salt behind.
O The procedure will make the soup less salty because
the salt will evaporate and leave the pot.
The saltiness of the soup will not change because
both salt and water will evaporate.
O The heat will cause the soup to become more salty
because more salt dissolves in hotter water.
Answers
Answer: A! The soup will become saltier because evaporation removes the water and leaves the salt behind.
Explanation:
Just finished the test and got a 90%! <3
Answer:
A
Explanation:
What is the answer of number 11?

Answers
Hope this helps!
during one of the trials in this project, the initial weight of ethanol is 77 g and after the combustion, the final weight of ethanol is 10. what are the number of moles of ethanol consumed during the experiment? the molar mass of ethanol is 46.07 g/mol. report and round your answer to the first decimal place.
Answers
The number of moles of ethanol consumed during the experiment is
The combustion reaction is given as :
C₂H₅OH + 3O₂ -----> 2CO₂ + 3H₂O
the initial weight of ethanol = 77 g
the final weight of ethanol is = 10 g
initial moles = mass / molar mas
= 77 / 46.07
= 1.67 g
final moles = mass / molar mass
= 10 / 46.07
= 0.21 mol
the moles consumed = 1.67 - 0.21 = 1.46
thus, during one of the trials in this project, the initial weight of ethanol is 77 g and after the combustion, the final weight of ethanol is 10. the number of moles of ethanol consumed during the experiment. the molar mass of ethanol is 46.07 g/mol is 1.46 mol
To learn more about moles here
https://brainly.com/question/26416088
#SPJ4
Los 4 números cuánticos del oxígeno
Answers
identify the first step in preparing a spectrophotometer for use.
Answers
We have that the first step is to put the Power source into an on state,Thereby powering the Light point and the Spectrophotometer.
From the question we are told
identify the first step in preparing a spectrophotometer for use.
Generally
A SpectrophotometerThis is a device is out there to help scientist i the mostly in the field of chemistry.
This device is used to Know or arcertain particle with light consuming properties.
The Spectrophotometer is Mostly found in laboratories.
And usually in the use of a Spectrophotometer the first step is to put the Power source into an on state.Thereby powering the Light point and the Spectrophotometer
Therefore
The first step is to put the Power source into an on state,Thereby powering the Light point and the Spectrophotometer.
For more information on this visit
https://brainly.com/question/14379882
I NEED HELP, THANKS!
Using the Ideal Gas Law, PV = nRT, where R = 0.0821 L atm/mol K, calculate the volume in liters of oxygen produced by the catalytic decomposition of 25.5 g potassium chlorate according to the following reaction. The oxygen is collected at 2.22 atm and 25.44°C. Express your answer to the correct number of significant figures.

Answers
Answer:
\(\large \boxed{\text{3.45 L}}\)
Explanation:
We will need a balanced chemical equation with molar masses, so, let's gather all the information in one place.
Mᵣ: 122.55
2KClO₃ ⟶ 2KCl + 3O₂
m/g: 25.5
(a) Moles of KClO₃
\(\text{Moles of KClO}_{3} =\text{25.5 g KClO}_{3} \times \dfrac{\text{1 mol KClO}_{3}}{\text{122.55 g KClO}_{3}} = \text{0.2081 mol KClO}_{3}\)
(b) Moles of O₂
The molar ratio is 3 mol O₂:2 mol KClO₃
\(\text{Moles of O$_{2}$}= \text{0.2081 mol KClO}_{3} \times \dfrac{\text{3 mol O$_{2}$}}{ \text{2 mol KClO}_{3}} = \text{0.3121 mol O$_{2}$}\)
(c) Volume of O₂
We can use the Ideal Gas Law to calculate the volume of hydrogen.
pV = nRT
T = (25.44 + 273.15) K = 298.59 K
\(\rm V = \dfrac{nRT}{p}= \dfrac{\text{0.3121 mol $\times$ 0.0821 L$\cdot$atm$\cdot$K$^{-1}$mol$^{-1}\times$ 298.59 K}}{\text{ 2.22 atm}} = \textbf{3.45 L} \\\\\text{The volume of oxygen is $\large \boxed{\textbf{3.45 L}}$}\)
Answer:
Solution:-
The balanced equation:
2KClO3 (s) \rightarrow 2KCl (s) + 3 O2 (g)
Molar mass of KClO3 = 122.55 g/mol
Number of moles of KClO3 = (Mass of KClO3 / Molar mass of KClO3) = (25.5 g / 122.55 g/mol) = 0.2081 mol
From balanced equation, 2 mol of KClO3 produce 3 mol of O2. Or, 1 mol of KClO3 produces (3/2) mol of O2.
therefore, 0.2081 mol of KClO3 will produce = (3/2) × (0.2081) = 0.3121 mol of O2
Now, we have number of moles (n) of O2 = 0.3121 mol
Pressure (P) = 2.22 atm
Temperature (T) = 25.44°C = (273.15 + 25.44) K = 298.59 K
R (Gas constant) = 0.0821 L.atm/mol.K
Volume (V) of O2 = ?
Using the ideal gas equation,
V = nRT / P
Substituting the values in the equation, we get :
V = (0.3121 mol × 0.0821 L.atm/mol.K × 298.59 K) / (2.22 atm) = 3.45 L
Hence, the volume of O2 gas produced = 3.45 L
Explanation:

how is the pollination of plants dependent on pollinators
Answers
The Process of Animal Pollination. Pollinators come to flowers in search of food (nectar and pollen). During a flower visit, a pollinator may unintentionally brush up against the flower's reproductive parts, depositing pollen from another flower. The pollen is then used by the plant to produce a fruit or seed.
fill in the blank. "Of the following, a 0.1 M aqueous solution of __________ will have the lowest freezing point.
a. NaCl
b. Al(NO3)3
c. Na2SO4
d. K2CrO4
e. sucrose"
"b. Al(NO3)3
more moles = lower boiling point"
Answers
I apologize for the mistake in my previous response. The correct answer is: "Of the following, a 0.1 M aqueous solution of ______ will have the lowest freezing point.
a. NaCl b. Al(NO3)3 c. Na2SO4 d. K2CrO4 e. sucrose" The answer is d. K2CrO4 because it will dissociate into three ions in solution, resulting in the greatest number of particles, which will lower the freezing point the most. The presence of more moles of a solute does not necessarily affect the freezing point, but it does affect the boiling point.
b. Al(NO3)3
In a 0.1 M aqueous solution of Al(NO3)3, there will be more moles of particles present compared to the other options, resulting in a lower freezing point due to the colligative property of freezing point depression.
To know more about Previous click here .
brainly.com/question/14919895
#SPJ11
in which change does oxidation occur? a. ch3 cho → ch3 ch2oh b. cro42-→ cr2o72- c. so42-→ so3 2- d. no2- → no3 -
Answers
In the given options, oxidation occurs in option D: NO2- → NO3-. In this change, the nitrogen atom increases its oxidation state from +3 in NO2- to +5 in NO3-, which indicates an oxidation process.
The chemical processes in which electrons are transferred from one chemical to another. Redox reactions, also known as oxidation-reduction reactions, are the name given to these electron-transfer processes. Energy changes in the form of heat, light, electricity, etc. accompany these reactions. The addition of oxygen or hydrogen to various substances is another step in the oxidation and reduction reactions.
In the given options, oxidation occurs in option D: NO2- → NO3-. In this change, the nitrogen atom increases its oxidation state from +3 in NO2- to +5 in NO3-, which indicates an oxidation process.
Visit here to learn more about Redox reactions : https://brainly.com/question/13293425
#SPJ11
"In which change does oxidation occur?"
The correct answer is option D: NO2- → NO3-.
In this change, the nitrogen atom in the nitrite ion (NO2-) is oxidized to form the nitrate ion (NO3-). Here's a step-by-step explanation:
1. Identify the oxidation states of nitrogen in both ions.
In NO2-, the nitrogen atom has an oxidation state of +3.
In NO3-, the nitrogen atom has an oxidation state of +5.
2. Compare the oxidation states.
The oxidation state of nitrogen increases from +3 in NO2- to +5 in NO3-.
3. Determine if oxidation occurred.
Since the oxidation state increased, oxidation occurred in this change.
So, the change in which oxidation occurs is NO2- → NO3-.
To know more about oxidation states:
https://brainly.com/question/31544275
#SPJ11
2. How many moles of carbon dioxide gas are in 0.667 liters?
Answers
0.034 mol of carbon dioxide gas, are there in 0.667 L.
We can assume that the volume of one mole of any gas is 22.4 L we know that the pressure of the gas is 1 atmosphere and that the temperature of a gas is 0C
This type is called standard temperature and pressure (STP).
This can be calculated by using the formula in which we can find the moles:
PV =nRT------(1)
Where P is the pressure
V is volume
T is the temperature in Kelvin
R is the ideal gas constant consistent with the units of volume and pressure.
0.667 L x 1 mol/22.4 L = 0.034 mol
And 0.667 liters of CO2 or any other gas at STP would contain 0.034 moles.
If the gas was at some other temperature or pressure, assuming they were “moderate,” you could calculate the number of moles by solving the following formula for n.
To Know more about Ideal gas:
https://brainly.com/question/12742620
6.0 mol NaOH can form
3.0 mol Na3PO4 while 9.0 mole H3PO4
can form 9.0 mol Na3PO4. What mass of
Na3PO4 forms?
Na3PO4: 164 g/mol
[?] g Na3PO4
Round your answer to the ones place.
g NasPO4
Answers
Answer:
1) 492 grams Na3PO4
2) 1,476 grams Na3PO4
Explanation:
6.0 mol NaOH forms 3.0 mol Na3PO4
9.0 mole H3PO4 forms 9.0 mol Na3PO4.
What mass of Na3PO4 forms?
1) 6.0 moles of NaOH
3.0 moles of Na3PO4 are formed. Convert thism into grams using the molar mass conversion factor: 164 g/mole
(3.0 moles Na3PO4)*(164 g/mole Na3PO4) = 492 grams
2) 9.0 moles of H3PO4
9.0 moles of Na3PO4 are formed. Again, use the molar mass conversion factor.
(9.0 moles Na3PO4)*(164 g/mole Na3PO4) = 1,476 grams Na3PO4
Warner likes waffles for breakfast how much energy is used by a waffle maker that has a power rating of. 7501kW and is operated for 3. 6h
Answers
Answer: 97,212,960,000 J/s
Explanation:
Power = Energy ÷ Time
So,
Energy = Time × Power
First convert 3.6 hrs into seconds
3.6 × 60
216 mins
216 × 60 = 12,960 seconds.
Convert 7501kW into Watts.
7501 × 1000 = 7,501,000
Substitute the values:
Energy = 12,960 × 7,501,000
Energy = 97,212,960,000 Joules per second.
Big number. But you did leave a 7501 kilo watt appliance running for over 3 hours..
Which of these elements will successfully create a 1:1 bond with magnesium
a: calcium
b: aluminum
c: silicone
d: sulfur
Answers
Out of the options given, calcium (a) is the element that will successfully create a 1:1 bond with magnesium.
Calcium and magnesium belong to the same group on the periodic table, known as Group 2 or the alkaline earth metals. These elements have two valence electrons, and they tend to form ionic compounds by losing these electrons to achieve a stable electron configuration.
When magnesium (Mg) loses two electrons, it forms a 2+ cation (Mg2+). Similarly, calcium (Ca) also loses two electrons to form a 2+ cation (Ca2+). Since both ions have the same charge, they can attract each other and form a 1:1 bond.
On the other hand, aluminum (b), silicone (c), and sulfur (d) do not have the same charge as magnesium. Aluminum forms a 3+ cation (Al3+), silicone forms a 4- anion (Si4-), and sulfur forms a 2- anion (S2-). These different charges make it unlikely for these elements to form a 1:1 bond with magnesium.
Therefore, calcium is the element that will successfully create a 1:1 bond with magnesium.
Learn more about magnesium here:-
https://brainly.com/question/13153254
#SPJ11
Aluminum has been studied as a factor that may be linked to: A. Alzheimer's disease B. Chronic renal disease C. Low blood pressure D. Melanosis of the skin E. None of the above
Answers
Aluminum has been studied as a potential factor linked to Alzheimer's disease . Option (A) is correct.
Numerous studies have been conducted to investigate the connection between aluminum exposure and the development of Alzheimer's disease, a neurodegenerative disorder characterized by memory loss and cognitive decline. Although no conclusive evidence has been found to establish a direct causal relationship between aluminum and Alzheimer's disease, some studies suggest that high aluminum levels in the brain may contribute to the formation of beta-amyloid plaques, which are characteristic of Alzheimer's disease.
However, aluminum has not been found to be linked to chronic renal disease (B), low blood pressure (C), or melanosis of the skin (D). These conditions have different causes and risk factors that are unrelated to aluminum exposure. Therefore, the correct answer is E. None of the above.
Learn more about Alzheimer's disease from:
https://brainly.com/question/27414232
#SPJ11
which part of the surface tension vs log concentration plot is indicative of the adsorption density of the surfactant at the air - liquid interface?
Answers
The adsorption density of the surfactant at the air-liquid interface is indicated by the steepest part of the surface tension vs log concentration plot.
What is adsorption density?Adsorption density is a measure of the amount of a substance that is adsorbed onto the surface of a material. It is typically expressed as the amount of substance adsorbed per unit area of the material surface. The adsorption density is important in determining how much of a substance can be adsorbed onto a surface, and is used in the design of materials used for storing and separating substances. Adsorption density can be affected by several factors, such as temperature, surface area, the type of material, and the presence of other substances.
This is because the steepest part of the plot indicates the highest concentration of the surfactant at the interface, which in turn is indicative of the highest adsorption density of the surfactant.
To learn more about steepest
https://brainly.com/question/30592456
#SPJ1
Which phrase best defines a covalent bond?
A. Weak polar attraction
B. Strong polar attraction
C. Electrons shared between atoms
D. Electrons transferred between atoms
Answers
This becouse In covalent bonding electrons are shared between atoms rather than donated in order for the atoms of both elements to gain full outer shells. Electrons are always shared in pairs. Example: An example of covalent bonding is the molecule of carbon dioxide
A phrase which best defines a covalent bond is: C. Electrons shared between atoms.
A chemical bond can be defined as the forces of attraction that exists between ions, crystals, atoms or molecules of chemical elements and they are typically responsible for the formation of new chemical compounds.
Hence, a chemical bond is simply a force holding atoms together and binding ions, crystals, or molecules of chemical elements together, in order to form a new chemical compound.
Basically, there are three (3) main types of chemical bonds and these are:
Ionic bonds.Hydrogen bonds. Covalent bonds.A covalent bond can be defined as a type of bond that typically involves the sharing of electrons between the atoms of a chemical element.
For example, the type of bond formed between two or more carbon atoms is a covalent bond.
In conclusion, a phrase which best defines a covalent bond is electrons shared between atoms.
Read more: https://brainly.com/question/24212500
decide which element probably has a boiling point most and least similar to the boiling point of strontium.
Answers
The boiling point of an element is largely determined by its intermolecular forces, which in turn are affected by factors such as atomic size, electronegativity, and the number of electrons.
In the case of strontium, which has a boiling point of 1382°C, the element with the most similar boiling point is likely to be one that is in the same group as it on the periodic table. This is because elements in the same group tend to have similar electronic configurations and atomic radii. Therefore, barium, which is in the same group as strontium, is likely to have a boiling point that is most similar. On the other hand, elements in different groups will likely have very different boiling points. For example, fluorine, which is in a different group than strontium, will likely have a boiling point that is least similar to that of strontium.
On the other hand, a non-metal element from a different group, like fluorine (F) in Group 17 and Period 2, would have a boiling point least similar to strontium due to the significant difference in their chemical properties and atomic structures.
To know about intermolecular:
https://brainly.com/question/31980535
#SPJ11
what is the stock system name for N2O3, CO, SO3
Answers
Answer:
N2O3= Dinitride Trioxide
CO= Carbon Oxide
SO3= Sulfide Trioxide
Explanation:
Di= 2
Tri= 3
Pls Help ASAP! 2 grams of potassium (K) reacts with 5 grams of Oxygen (O). According to the Law of Conservation of Mass, how many grams of potassium oxide (K2O) will be produced?
O 10 grams
O 7 grams
O 2 grams
O 5 grams
Answers
Taking into account the reaction stoichiometry, 2.4041 grams of K₂O can be produced from 2 grams of K and 5 grams of O₂.
Reaction stoichiometryIn first place, the balanced reaction is:
4 K + O₂ → 2 K₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
K: 4 molesO₂: 1 mole K₂O: 2 molesThe molar mass of the compounds is:
K: 39.1 g/moleO₂: 32 g/moleK₂O: 94.2 g/moleThen, by reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
K: 4 moles ×39.1 g/mole= 156.4 gramsO₂: 1 mole ×32 g/mole= 32 gramsK₂O: 2 moles ×94.2 g/mole= 188.4 gramsLimiting reagentThe limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
Limiting reagent in this caseTo determine the limiting reagent, it is possible to use a simple rule of three as follows: if by stoichiometry 32 grams of O₂ reacts with 156.4 grams of K, 5 grams of O₂ reacts with how much mass of K?
mass of K= (156.4 grams of K× 5 grams of O₂)÷ 32 grams of O₂
mass of K= 24.4375 grams
But 24.4375 grams of K are not available, 2 grams are available. Since you have less mass than you need to react with 5 grams of O₂, K will be the limiting reagent.
Mass of K₂O formedConsidering the limiting reagent, the following rule of three can be applied: if by reaction stoichiometry 156.4 grams of K form 188 grams of K₂O, 2 grams of K form how much mass of K₂O?
mass of K₂O= (2 grams of K× 188 grams of K₂O)÷ 156.4 grams of K
mass of K₂O= 2.4041 grams
Then, 2.4041 grams of K₂O can be produced from 2 grams of K and 5 grams of O₂.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
Answer:
(Question) What does the Law of Conservation of Mass state?
(Answer) The total mass of all of the reactants prior to a chemical reaction must equal the total mass of all the products after the reaction.
(Question) If the mass of elements before a chemical reaction is 30 grams, after the chemical reaction, the mass will be __.
(Answer) 30 grams
(Question) 78 g of potassium (K) react with 71 g of chlorine (Cl) to produce potassium chloride. According to the Law of Conservation of Mass, what is the mass of the product (2KCl)?
(Answer) 149 g
(Question) 2 grams of potassium (K) reacts with 5 grams of Oxygen (O). According to the Law of Conservation of Mass, how many grams of potassium oxide (K2O) will be produced?
(Answer) 7
(Question) Which of the following equations demonstrates the Law of Conservation of Mass?
(Answer) CH4+O2→C+2H2O
Explanation:
just finished the quick check. hope this helps UwU
Which compound is an organic acid?
A.CH3OH
B.CH3OCH3
C.CH3COOH
D.CH3COOCH3
Answers
Answer:
C
Explanation:
CH3COOH
ethanoic acid
Answer:
C: CH3COOH
Explanation:
An organic acid (acetic acid) - a carboxylic acid has a functional group of:
R - COOH
Which type of PPE is designed to shield or isolate a responder from chemical or biological hazards?
Select one:
a.Chemical-protective clothing (CPC)
b.Flame-resistant protective clothing
c.High temperature-protective clothing
d.Structural firefighters' protective clothing
Answers
Chemical-protective clothing (CPC) is designed to shield or isolate a responder from chemical or biological hazards.
Chemical-protective clothing (CPC) is specifically designed to shield or isolate a responder from chemical or biological hazards. It is made of specialized materials that provide a barrier against hazardous substances, preventing them from coming into contact with the wearer's skin or clothing. This type of PPE is essential in situations where there is a risk of exposure to dangerous chemicals or biological agents.
Therefore, option a.Chemical-protective clothing (CPC) is correct.
To learn more about Chemical-protective clothing (CPC), Visit:
https://brainly.com/question/6547716
#SPJ11
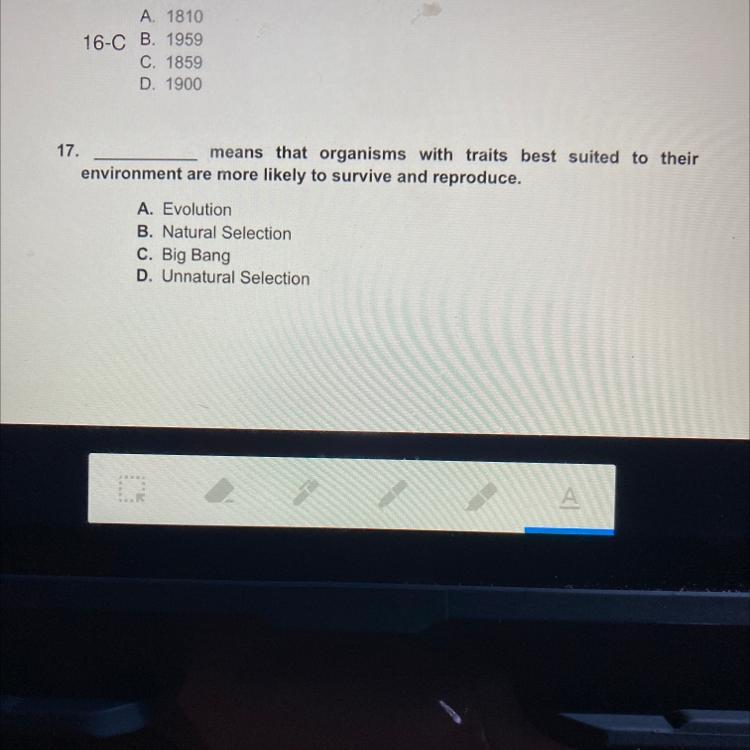
I need the answer for number 17 pls
Answers
QUESTION: What is the main function (job) of the Nervous System?
A. The nervous system controls the breakdown of food into small molecules, which are then absorbed into the body.
B. The nervous system's main job is to move fresh air into your body while removing waste gases.
C. The nervous system controls everything you do, including breathing, walking, thinking, and feeling.
D. The nervous system carries oxygen, nutrients, and hormones to cells, and removes waste products, like carbon dioxide.
Answers
Answer:
It is C
Explanation:
it sends signals to the brain so it would be C
Answer:
C. The nervous system controls everything you do, including breathing, walking, thinking, and feeling.
is the answer
Calculate the number of nitrogen atoms in 20.9 grams of nitrogen.
Answers
Answer:
N=5.47×10^23
Explanation:
n=
\(n = \frac{m }{ar} \)
N=n×la
Can someone help me

Answers
Answer:
the third option
Explanation:
after you choose that answer don't check if it's correct
◑﹏◐
What characteristics would show that this cat and kitten are related? Support your answer by explaining two inherited characteristics.

Answers
1. A cat and it’s litter can’t be related without the producer which in this case is the mother
2. The stripes shows that the two are related and yeah you can have to cats that have the same stripes but that’s not likely, every-time any two cats with stripes are put together the cats show similarities but that doesn’t tell us that their related. Every cat has similar stripes but there is something that’s always gonna be off with the stripes whether the stripes have the same color or the stripes have the same arrangement on the cat. Like people cats have similar traits but that doesn’t mean there related (ex: two blue eyed people, that’s doesn’t prove that their related in any type of way). Hopes this help!
The liver is a group of tissues that produce bile that is used to break down fats. The liver is a(n) _____.
Answers
Answer:
Its food for cannibals.
in the correct lewis structure for ch4 how many unshared electron pairs surround the carbon?
Answers
In the correct Lewis structure for CH4 (methane), there are no unshared electron pairs surrounding the carbon atom.
Methane consists of a carbon atom bonded to four hydrogen atoms. Each hydrogen atom shares one electron with the carbon atom, forming four sigma bonds. Carbon has four valence electrons, and each hydrogen contributes one valence electron, resulting in a total of eight valence electrons in the molecule.
To represent this structure in a Lewis structure, we place the carbon atom in the center and connect it to the four hydrogen atoms through single bonds. The carbon atom is surrounded by four regions of electron density, which are the four sigma bonds. Since there are no unshared electron pairs around the carbon atom, it follows the octet rule, achieving a stable configuration.
Therefore, in the correct Lewis structure for CH4, the carbon atom is surrounded by four sigma bonds, with no unshared electron pairs.
Know more about Lewis Structure here:
https://brainly.com/question/29603042
#SPJ11
A student mixes 40. mL of 0.10 M HBr(aq) with 60. mL of 0.10 M KOH(aq) at 25°C. What is the [OH-] of the resulting solution?
Answers
The [OH⁻] of the resulting solution after mixing 40 mL of 0.10 M HBr(aq) with 60 mL of 0.10 M KOH(aq) at 25°C is 0.020 M.
To find the [OH⁻] of the resulting solution after mixing 40 mL of 0.10 M HBr(aq) with 60 mL of 0.10 M KOH(aq) at 25°C, first, determine the moles of HBr and KOH:
Moles of HBr = volume × concentration = 0.040 L × 0.10 mol/L = 0.004 mol
Moles of KOH = volume × concentration = 0.060 L × 0.10 mol/L = 0.006 mol
Since HBr and KOH react in a 1:1 ratio, 0.004 moles of HBr will react with 0.004 moles of KOH, leaving 0.002 moles of unreacted KOH.
Next, find the total volume of the solution:
Total volume = 0.040 L + 0.060 L = 0.100 L
Now, calculate the concentration of OH⁻ ions:
[OH⁻] = moles of unreacted KOH / total volume = 0.002 mol / 0.100 L = 0.020 M
So, the [OH⁻] of the resulting solution is 0.020 M.
Learn more about resulting solution: https://brainly.com/question/31358971
#SPJ11