The first atomic structure *
Yes
NO
Answers
Answer:
Yes? Sry I’m kinda confused
Explanation:
Sry if I’m wrong
Related Questions
The different possible ways for arranging the particles of a system are called _____. The greater the number of these states, the _____ the entropy of the system
Answers
The different possible ways of arranging the particles of a system are called states. The greater the number of these states, the higher the entropy of the system.
By ascribing definite values to a satisfactory amount of variables, one can define the state of a system. In simple terms, it is the description of a system condition in terms of properties that are measurable or observable, for example, pressure, temperature, etc.
Entropy is a measure of the disorder or randomness in a system, and an increase in the number of states corresponds to an increase in entropy. The S.I. unit for entropy is joules per kelvin. Entropy is a measurable physical property. In a thermodynamic system, it is an extensive property.
Example: There is an increase in entropy when a block of ice melts.
To learn more about entropy, visit: https://brainly.com/question/15025401
#SPJ11
clerice midter
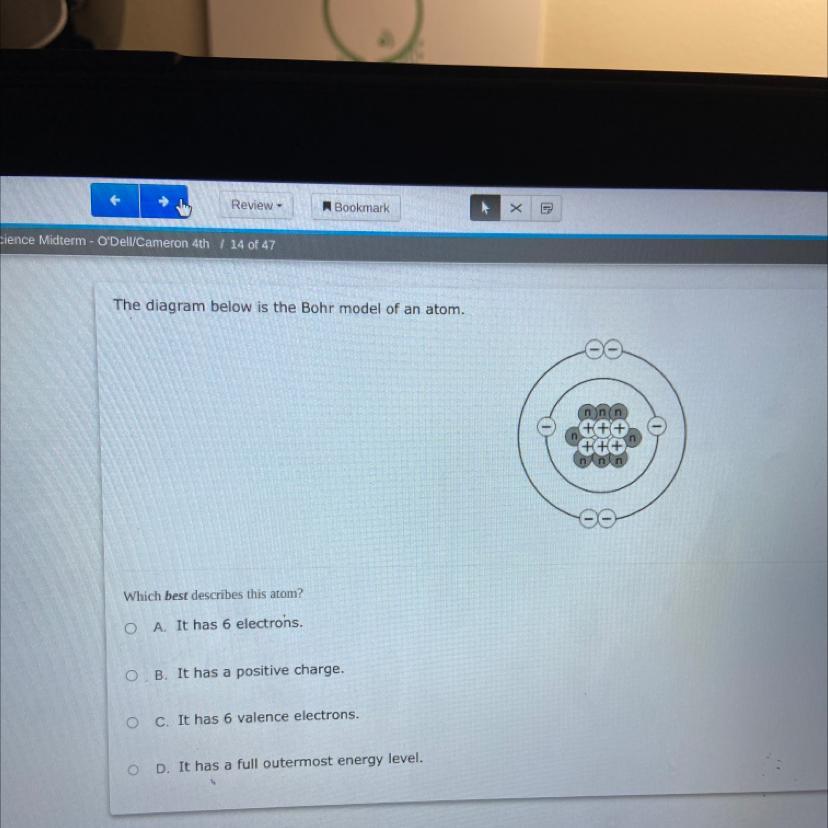
The diagram below is the Bohr model of an atom.
Which best describes this atom?
OA. It has 6 electrons.
OB. It has a positive charge.
O c. It has 6 valence electrons.
OD.
has a full outermost energy level.
Answers
The correct option is (A) - This Bohr Model of atom describes that there are a total of 6 electrons in the given figure.
What is Bohr Model of atom?The electrons are positioned in circular orbitals at particular distances from the central nucleus in the Bohr model of the atom. These orbits create electron shells or energy levels, which allow us to see how many electrons are present in each shell. The number and the letter "n" are used to identify these energy levels. The first energy level nearest to the nucleus, for instance, is represented by the 1n shell. Normally, an electron resides in the shell with the lowest energy, which is the one closest to the nucleus. A photon of light's energy can raise it to a higher energy shell, but this is an unstable position, and the electron quickly returns to the ground state.
Learn more about atom here:
https://brainly.com/question/30898688
#SPJ1
Write The molecular formula Of The following substances in the given pattern
1. ferrous oxide
2. silicon chloride
3. barrium nitrate
4. calcium hydroxide
Answers
Answer:
1. FeO
2.
3. Ba(NO3)2
4. Ca(OH)²
Explanation:
sorry I didn't knew of no 2 the others were on my book but not that one, and of no 4 the power 2 must be down
*The diagram has to do with the question*
Pls help

Answers
Wgsystststsgdhdb
A reaction that releases energy is called endothermic .
True or False
Answers
Answer: False
Explanation: Endothermic means it absorbs heat energy.
explain how shielding contributes to the atomic radius trend within a group.
Answers
Shielding is a term used to describe the ability of inner electrons to shield or reduce the effective nuclear charge (Zeff) experienced by the valence electrons in an atom.
The number of valence electrons and the outermost energy level (also known as the primary quantum number or n value) are the same for all elements in a periodic table group (or column). This indicates that the valence electrons in all of the group's atoms have comparable energy levels and shielding effects from the inner electrons. As a result, the number of energy levels, or the primary quantum number (n), and the shielding effect of the inner electrons dictate the trend in atomic radius within a group.
For such more question on atom:
https://brainly.com/question/26952570
#SPJ4
DNA can be found on long strands of?
Answers
Answer:
DNA bases pair up with each other, A with T and C with G, to form units called base pairs. Each base is also attached to a sugar molecule and a phosphate molecule. Together, a base, sugar, and phosphate are called a nucleotide. Nucleotides are arranged in two long strands that form a spiral called a double helix.
What does the formula ΔH − TΔS determine?
entropy
Le Châtelier’s principle
enthalpy
Gibbs free energy
Answers
please answer this question fast!

Answers
Answer:
The answer is C for sure!
Explanation:
In the picture we have the electrons given, so we have to determine the nucleons and protons.
Draw the Lewis dot structure for RbF
Answers
There are several steps to draw lewis dot structure of any chemical compound. Here, we taking RbF. Structure is given below with the image.
1. Start by writing the chemical symbol for the element at the center of the structure. In this case, it is Rb for Rubidium.
2. Draw dots around the symbol to represent the valence electrons. Rb has one valence electron, so you would put one dot around the Rb.
3. Next, write the chemical symbol for the other element in the compound, in this case F for Fluorine.
4. Draw dots around the symbol to represent the valence electrons. Fluorine has 7 valence electrons, so you would put 7 dots around the F.
5. To create the bond between the two elements, use a pair of dots to connect the Rb and F. This represents the sharing of electrons between the two elements.
So the Lewis dot structure for RbF would be represented as:
. .
Rb+ [:F:]
. .
This representation shows that Rb and F share one pair of electrons, forming a bond between them, and the dots around Rb and F represent the remaining number of valence electrons that are not involved in the bond.
To know more about RbF please refer: https://brainly.com/question/13526282
#SPJ4

Name a non metal which conducts electricity
Answers
Answer:
Graphite
Graphite is a non-metal and it is the only non-metal that can conduct electricity.
Calculate the standard reaction enthalpy for the reaction below:
3Fe2O3(s) → 2Fe3O4(s) + ½O2(g)
Answers
The standard reaction enthalpy for the given reaction is +235.8 kJ/mol.
What is the standard reaction enthalpy of reaction?The standard reaction enthalpy (ΔH°) for the given reaction is determined as follows:
Equation of reaction: 3 Fe₂O₃ (s) → 2 Fe₃O₄ (s) + ½ O₂ (g)
The standard enthalpy of formation values for Fe₂O₃ (s), Fe₃O₄(s), and O₂(g) is used to calculate the standard reaction enthalpy.
ΔH° = [2 × ΔH°f(Fe₂O₃)] + [½ × ΔH°f(O₂)] - [3 × ΔH°f(Fe₃O₄)]
where;
ΔH°f(Fe₂O₃) = -824.2 kJ/mol
ΔH°f(Fe₃O₄) = -1118.4 kJ/mol
ΔH°f(O₂) = 0 kJ/mol
ΔH° = [2 × (-1118.4 kJ/mol)] + [½ × 0 kJ/mol] - [3 × (-824.2 kJ/mol)]
ΔH° = -2236.8 kJ/mol + 0 kJ/mol + 2472.6 kJ/mol
ΔH° = 235.8 kJ/mol
Learn more about standard reaction enthalpy at: https://brainly.com/question/15174388
#SPJ1
What does this demonstration show?
Answers
Elements can be described by various properties, and identified by their boiling and melting points. For example, gold melts at 1,064ºC and boils at 2,856ºC. Does boiling point depend on the mass present?
A. No; chemical properties stay the same regardless of mass.
B. No; physical properties stay the same regardless of mass.
C. Yes; physical properties can change when mass increases or decreases.
D. No; qualitative properties like boiling point stay the same regardless of mass.
Answers
Answer:
Explanation:
Melting and boiling point variations are not clear (do not have uniform pattern) in periodic table. But we can see, some elements have higher melting and boiling points and some have less. Here we study melting and boiling points of s, p, d blocks elements. IVAth group elements (C,Si) show high melting and boiling points because they have covalent gigantic lattice structures.
calculate the concentration of the following solutions after being diluted to a final volume of 25 ml: a. 1.00 ml of 0.452 m na
Answers
The concentration of solution after dilution comes out to be 0.113 M as shown in the below section.
Using the dilution law, the concentration of the solution can be calculated as follows-
M₁ x V₁ = M₂ x V₂ ......(1)
Here, M signifies the concentration and V represents the volume.
It is given,
V₁ = 25 mL
V₂ = 100 mL
M₁ = 0.452 M
To calculate the concentration/molarity of solution on dilution, substitute the above values in the equation (1) as follows-
0.452 M x 25 mL = M2 x 100 mL
M2 = (0.452 M x 25 mL) / 100 mL
M2 = 0.113 M
Therefore, the concentration of solution after dilution comes out to be 0.113 M.
To learn more about molarity check the link below-
https://brainly.com/question/30404105
#SPJ4
Convert 3.01×10 23 molecules of H2O to moles.
Answers
In a solution of brine (salt water), what is the salt? A. a solute B. a solvent C. a solution D. a reactant E. a product
Answers
Answer:
solute
Explanation:
solvent is the water solution is the whole mixture product is the solution
A. a solute
B. a solvent
C. a solution
D. a reactant
E. a product
All of the following statements about lighteners are true EXCEPT:a) they are used at relatively high levelsb) they slowly block the production of melanin in the skinc) they are used to bleach or lighten areas of hyperpigmentationd) they usually take months of continued use to deliver a noticeable effect
Answers
With the exception of (a), all of the following statements regarding lighteners are true.
What causes hyperpigmentation?Melanin, a pigment made by skin cells, is what gives skin their colour. The skin cells may produce far to much melanin if they are sick or injured. There might be a melanin deposition there, darkening it up. Fading could take a while after the dark spots and patches' root causes have been identified and eliminated. An area that is just a few shades darker than you natural skin color takes 6 to 12 months to heal on average. Blue-gray-appearing hyperpigmentation in the dermis may last a long time or become permanent if addressed.
Which serum is best for hyperpigmentation?Lightweight Anti-Pigmentation Serum: Clinical studies have shown that the advanced skin-lightening ingredient Alpha Arbutin, which is included in this de pigmentation serum, reduces hyperpigmentation and blemishes.
To know more about Hyperpigmentation visit:
https://brainly.com/question/14567659
#SPJ4
Please help I'm struggling
What type of attractive force exists when two atoms form a temporary dipole or charge because of the movement of electrons?

Answers
Answer:
The correct option is A
Explanation:
The forces of attraction among the molecules or atoms of a substance are called inter molecular forces.
When two atoms gain temporary dipole and become polar for an instant the inter molecular forces of attraction between then are known as London dispersion forces.
London Dispersion Forces:
It is a type of inter molecular force that occurs when the electrons in two adjacent atoms are displaced in such a way that the atoms get some temporary dipoles, they attract each other through London dispersion forces. These inter molecular forces usually occur between non polar substances.
Answer: B intramolecular force
Explanation:
Which one of the following is least likely to be an elementary step in a reaction mechanism?NO2(g) + O3(g) → NO3(g) + O2(g)C2H6(g) + 5O2(g) → 2 CO2(g) + 3 H2O(g)2NO(g) + O2(g) → 2 NO2(g)2HI(g) → H2(g) + I2(g)NO3(g) + NO(g) → 2 NO2(g)
Answers
The second reaction, C2H6(g) + 5O2(g) → 2 CO2(g) + 3 H2O(g), is least likely to be an elementary step in a reaction mechanism. This is because it involves the simultaneous collision of seven molecules, which is very unlikely to occur in a single step.
Elementary steps usually involve the collision of one or two molecules, or the breaking or formation of a bond between two atoms or molecules. The other reactions listed all involve one or two molecules colliding, or the breaking or formation of a bond between two atoms or molecules, and are more likely to be elementary steps in a reaction mechanism.
This is because the probability of multiple molecules colliding simultaneously is lower than that of a single molecule colliding with another molecule.
Therefore, the second reaction listed, C2H6(g) + 5O2(g) → 2 CO2(g) + 3 H2O(g), is least likely to be an elementary step in a reaction mechanism due to the large number of particles involved in the collision. Instead, this reaction is likely to proceed through a series of multiple elementary steps that involve the sequential breaking and formation of multiple bonds between atoms and molecules.
Learn more about reaction mechanism here:
https://brainly.com/question/26690612
#SPJ4
1. A calcium chloride hydrate, cacl2. Xh2o, has a mass of 4. 72 g. After heating for several minutes, the mass of the anhydrous is found to be 3. 56 g. Find the molecular formula of the hydrate
Answers
The molecular formula for hydrate has been, CaCl₂.2H₂O.
What is the molecular formula?
The molecular formula is given as the representation of the compound with the number of atoms of each element.
The hydrate has a mass of 4.72 g. The anhydrous has a mass of 3.56 g. The mass of water will be:
Mass of water = Mass of hydrate - Mass of anhydrate
Mass of water = 4.72 g - 3.56 g
Mass of water = 1.18 grams
The moles of calcium chloride in 4.72 grams are:
Moles = Mass/ Molar mass
Moles of Calcium chloride = 4.742 g / 110.98 g/mol
Moles of Calcium Chloride = 0.0321 mol
The moles of water in 1.18 grams are :
Moles of water = 1.18 g / 18 g/mol
Moles of water = 0.0655 mol
The ratio of calcium chloride to water in the compound will be:
Calcium chloride / water = 0.0321 / 0.0655
Calcium chloride / water = 2 / 1
Thus, the molecular formula for hydrate has been, CaCl₂.2H₂O.
Learn more about molecular formulas, here:
https://brainly.com/question/14425626
#SPJ4
SO3(g) + H2O(l) → H2SO4(aq)
Calculated mass of SO3 used: 40. 1g
Volume of H2O used: 10mL
Concentration of the produced H2SO4: 4. 9M
Volume of produced H2SO4: 20. 1mL
Density of water: 1g/cm3
What is the yield of this reaction?
Answers
The yield of the response is 245.6% and the number of moles of H2SO4 is 0.09849 moles.
To decide the yield of the response, we first need to ascertain how much \(H2SO4\) created:
Volume of H2SO4 = 20.1 mL = 0.0201 L
Grouping of H2SO4 = 4.9 M
Measure of H2SO4 = \(fixation x volume\) = \(4.9 M x 0.0201 L = 0.09849\) moles
We realize that the response is:
\(SO3(g) + H2O(l) → H2SO4(aq)\)
The decent condition lets us know that 1 mole of SO3 responds with 1 mole of H2O to create 1 mole of H2SO4. Hence, the hypothetical yield of \(H2SO4\) would be 0.0401 moles (since 40.1g of SO3 is comparable to 0.4 moles of SO3).
The genuine yield is given \(H2SO4\) created, which is 0.09849 moles.
The yield of the response is then, at that point:
Yield = \((genuine yield/hypothetical yield) x 100 percent\)
= \((0.09849 moles/0.0401 moles) x 100%\)
= 245.6%
Subsequently, the yield of the response is 245.6%.
To learn more about yield, refer:
https://brainly.com/question/9809198
#SPJ4
Given the following equation: 2 AgNO3 CaCl2 --> 2 AgCl(s) Ca(NO3)2 What is the net ionic equation:
Answers
The net ionic equation is:
2Ag⁺(aq) + 2Cl⁻(aq) → 2AgCl(s)
How to write a net ionic equation?The given equation is:
2AgNO₃ (aq) + CaCl₂ (aq) → 2AgCl(s) + Ca(NO₃)₂ (aq)
A double displacement reaction occurs when silver nitrate and calcium chloride are combined. Silver chloride precipitate and a calcium nitrate solution are the results of the reaction.
Break down all the soluble electrolytes, that are present in an aqueous form, into their respective ions:
2Ag⁺(aq) + 2NO³⁻(aq) + Ca²⁺(aq) + 2Cl⁻(aq) → 2AgCl(s) + Ca²⁺(aq)+ 2NO³⁻(aq)
Now remove all the spectator ions present in the equation. The spectator ions should be removed after comparing the reactant and product sides of the revised reaction. These dissolved ions are known as spectator ions if they exhibit the same form on both sides. No reaction occurs if everyone is a spectator ion.
Now write the net ionic equation:
2Ag⁺(aq) + 2Cl⁻(aq) → 2AgCl(s)
Learn more about ionic equations here:
https://brainly.com/question/1601524
#SPJ4
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
True or False? If friction didn’t exist, an object in motion would stay in motion forever.
Answers
Answer:
True
Explanation:
Friction is defined as the force that opposes motion when an object is sliding over a surface.
As a result of friction, all objects moving over a surface eventually come to rest over time.If we were to successfully create a friction-less surface, an object will remain in motion forever because it will encounter no opposition to its motion.
Hence, the resistance to the motion of objects over a surface which causes the objects to come to a halt after moving over the surface for some time is called friction.
3.125% of a radioactive sample is left. How many half-lives have passed?
4 half-lives
1 half-life
5 half-lives
2 half-lives
3 half-lives
Answers
There would be 5 half-lives
What is the half life of an isotope?The half-life of an isotope is the amount of time it takes for half of the atoms of a radioactive isotope to decay into another isotope or element. It is a characteristic property of a specific isotope and does not depend on the amount of the material present.
The half-life of a radioactive isotope is a constant that can be used to measure the age of minerals and rocks, as well as to track the progress of nuclear reactions and to diagnose and treat certain medical conditions.
Learn more about half life:https://brainly.com/question/24710827
#SPJ1
A rock's density is 5 G / cm3 determine the mass of the rock if it has a volume of 20cm3
Answers
Answer:
100 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volume
From the question
density = 5 g/cm³
volume = 20 cm³
We have
mass = 5 × 20 = 100
We have the final answer as
100 gHope this helps you
Which statement correctly explains how matter is conserved in chemical reactions? (1 point)
A. The number of atoms in the reactants is always equal to the number of atoms in the products.
B. The states of matter of the reactants are always the same as the states of matter of the products.
C. The number of reactants is always equal to the number of products.
D. The number of molecules in the reactants is always equal to the number of molecules in the products.
Answers
According to the Law of Conservation of Matter, matter cannot be created nor destroyed. This means when a chemical process is underway, matter can’t be destroyed or created during the cycle. Balanced chemical equations, such as photosynthesis, prove the Law of Conservation of Matter to be correct because the same number of atoms were sustained on both sides of the equation.
A photon has an energy of 2.88×10^-18 J . Convert energy into eV.
Answers
A photon has an energy of 2.88 × 10⁻¹⁸ J. The energy into eV is 17.97 eV.
What is Photon Energy ?The energy which is carried by a single photon is called Photon energy.
It is expressed as:
E = hf
where,
E = Photon energy
h = Planck's energy
f = Wave frequency
How to convert Joules (J) into Electron volt (eV) ?To convert J into eV
E(eV) = E(J) × 6.241509 × 10¹⁸
= 2.88 × 10⁻¹⁸ × 6.241509 × 10¹⁸
= 17.97 eV
Thus from the above conclusion we can say that A photon has an energy of 2.88 × 10⁻¹⁸ J. The energy into eV is 17.97 eV.
Learn more about the Photon Energy here: https://brainly.com/question/19385998
#SPJ1
DANGERS OF ACIDS AND ALKALIS
Answers
Chemical substances with opposing characteristics include acids and alkalis. Alkalis have a pH above 7, while acids have a pH below 7.
What threats do acids pose?Acids are dangerous when there is moisture in the mouth, eyes, or surrounding aqueous solutions because they react violently with water. Certain acids' vapours can harm the eyes, nasal passages, throat, and lungs since they are soluble in water.
What poses the biggest threat to acids and bases?Working with acids and bases poses health risks mostly because of their corrosivity, which causes tissue to be destroyed. The pernicious characteristic of hydrofluoric acid, or HF, which causes severe loss of skin without providing any burning sensation.
To know more about Alkalis visit:-
https://brainly.com/question/18153051
#SPJ1
Question:
What are the potential dangers associated with acids and alkalis?