the first 32 amino acids from the N terminus of the protein bovine angiogenin were determined by edman degradation and have the sequence: AQDDYRYIHFLTQHYDAKPKGRNDEYCFNMMK (a) Identify the sites of cleavage during trypsin-catalyzed hydrolysis of this protein. (b) What are the cleavage sites using chymotrypsin?
Answers
The first 32 amino acids from the N terminus of the protein bovine angiogenin were determined by edman degradation. (a) The cleavage sites for the trypsin-catalyzed hydrolysis are, AQDDYRYIHFLTQHYDAKPKGRNDEYCFNMMK.
(b) The following cleavage sites take place during hydrolysis that is catalyzed by chymotrypsin: AQDDYRYIHFLTQHYCFNMMK. Site-selective breakdown of extremely non-reactive peptide bonds also functions as a therapeutically helpful modulator of protein structure and function, providing essential information on the protein sequence. Regulated and selective cleavage is a challenging task and requires chemical reagents to be able to recognize or bind to one or more specific amino acid residues in the peptide chain. On the basis of this concept, we developed a technique that selectively changes the serine residue in a peptide chain using a chemical reagent, causing the peptide backbone to break at the N-terminus of the serine residue. After cleavage, modified residues can be transformed back into their original form. This method may cleave a wide variety of substrates and does so specifically for certain bioactive peptides with post-translational modifications (like N-acetylation and -methylation) and mutations (like D- and -amino acids), which are known to contribute to age-related diseases.
To know more about cleavage please refer: https://brainly.com/question/9161095
#SPJ4
Related Questions
The ionic compound MX(s) is formed from the metal M(s) and the diatomic gas X2(g) at standard conditions. Calculate the lattice energy given the following data( data in picture)

Answers
The lattice energy of MX is 459.2 kJ/mol.
The lattice energy (ΔH° lattice) of an ionic compound is the energy released when one mole of the solid is formed from its constituent gaseous ions under standard conditions. The lattice energy is calculated using the Born-Haber cycle, which involves several steps including atomization, ionization, dissociation, and sublimation energies.
The lattice energy is related to the Coulombic attraction between the oppositely charged ions in the solid. To calculate the lattice energy for MX, we can use the following equation:
ΔH° lattice = ΔH° sub + ΔH° ion + ΔH° diss + ΔH° formation
where ΔH° sub is the sublimation energy of M(s), ΔH° ion is the first ionization energy of M(g), ΔH° diss is the dissociation energy of X2(g), and ΔH° formation is the enthalpy of formation of MX(s).
Using the given data, we can calculate each of these values and substitute them into the equation to obtain the lattice energy. The final answer should be in units of kJ/mol.
ΔH° sub (M) = 107.3 kJ/mol
ΔH° ion (M) = 577.5 kJ/mol
ΔH° diss (X2) = 242 kJ/mol
ΔH° formation (MX) = -467.6 kJ/mol
ΔH° lattice = 107.3 + 577.5 + 242 + (-467.6) = 459.2 kJ/mol
As a result, MX has a lattice energy of 459.2 kJ/mol.
To know more about the Ionic compound, here
https://brainly.com/question/1603676
#SPJ1
what are the colors of a rainbow
Answers
what element has Atom of 47
Answers
What are the answers to the following 5 multiple choice questions

Answers
The balanced equation of the reaction is option d. The limiting reactant here is KBr and excess reactant is calcium nitrate. The percent yield of the reaction is 93 %.
What is limiting reactant ?The limiting reactant in a reaction is the reactant which is fewer in amount and as soon it is consumed, the reaction stops. For the given reaction, option d is the balanced chemical equation.
One mole of calcium nitrate requires 2 moles of KBr.
molar mass of calcium nitrate = 164 g/mol
no.of moles in 75 g = 75/164 = 0.457
molar mass of KBr = 118.9 g/mol
no.of moles 95 g = 95/118.9 = 0.798
0.457 moles of calcium nitrate needs its twice amount that is 0.9 moles of KBr. Hence, KBr is the limiting reactant and calcium nitrate is excess reactant here.
2 moles or 237.8 g of KBr gives 2 moles or 202 g of potassium nitrate. Then, 95 g of KBr will gives:
(95×202)/237.6 = 81.3 g
actual yield = 75.75 g
then percent yield = 75.75 /81.3 × 100 = 93 %.
0.79 moles of KBr needs its half or 0.399 moles of calcium nitrate. But we have 0.45 moles. Thus, excess amount is
(0.45 - 0.399) × 164 g/mol = 9.5 g.
Therefore, the 9.5 g of excess reactant will be left over.
Find more on limiting reactants:
https://brainly.com/question/28938721
#SPJ1
You are asked to help design a bimetallic temperature sensor. You are given five metal alloys to work with; their thermal expansion coefficients are listed below. Assuming the elastic properties for each alloy are identical, which combination of alloys would give you the largest temperature-sensitive deflection for your sensor design?
Thermal Expansion Coefficients of Alloys
Alloy 1: 15 m/m-K
Alloy 2: 10 m/m-K
Alloy 3: 10 m/m-K
Alloy 4: 8 m/m-K
Alloy 5: 5 m/m-K
Alloy 1 and Alloy 2
Alloy 1 and Alloy 4
Alloy 1 and Alloy 5
Alloy 2 and Alloy 3
Alloy 4 and Alloy 5
Alloy 1 / Alloy 2 and Alloy 1 / Alloy 3 would give equally large deflections.
Alloy 1 and Alloy 5
Answers
The combination of alloys 1 and 5 would give the largest temperature-sensitive deflection for your bimetallic temperature sensor design. Thus, the correct option is C.
A bimetallic temperature sensor works by using two different metals with different thermal expansion coefficients that are bonded together. When the temperature changes, one metal will expand more than the other, causing the bond to bend. To achieve the largest temperature-sensitive deflection, it is important to use two metals with the largest possible difference in thermal expansion coefficients.
In this case, alloys 1 and 5 have the largest difference, with thermal expansion coefficients of 25 m/m-K and 2 m/m-K respectively, resulting in the largest deflection and making it the most suitable combination for a temperature sensor design.
Learn more about bimetallic stemmed thermometer here: brainly.com/question/30289652
#SPJ4
Suppose you have a radioisotope with a half-life of 2 years and you start with 800 grams. How much will you have two years from today? How much will you have 8 years from today?
Answers
8 years: 3.125
How many grams of sodium hydroxide are present in a 250.0 ml of a 0.600M NaOH solution?
Answers
0.06 grams
Hope it helps!
You just drove from Washington into Canada. You are driving a 1966 Ford Mustang. You notice the road sign says 100. Why are the other cars not speeding past you? Explain with a calculation, show your work. Remember 2.54 cm = 1 in.
Answers
Because the speed limit sign is in kilometres per hour and the driver is accustomed to miles per hour, no other cars are passing too quickly. The permitted speed is roughly 62 mph.
How do speed limit signs work?A regulatory sign is the speed limit sign. The purpose of speed limit signs is to inform drivers of the legal maximum and minimum speeds that are required of them. Drivers are not permitted to go over the limit stated by the sign.
What is the top speed at which a car can pass a procession?When approaching or passing a troop, police, or military procession, or when driving past construction workers fixing roads, a motor vehicle's driver must travel at a pace of no more than 25 kph.
To know more about limit visit:-
https://brainly.com/question/27439796
#SPJ1
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
How many moles of methane gas will combust to produce 330 grams of water? Answer must be to the correct number of sig figs and include the correct unit with the chemical formula.
Answers
9.162 moles of methane gas will combust to produce 330 grams of water.
What is the balanced chemical equation for the combustion of methane?The balanced chemical equation for the combustion of methane (CH₄) in the presence of oxygen (O₂) to produce water (H₂O) and carbon dioxide (CO₂) is:
CH₄ + 2O₂ -> CO₂ + 2H₂O
From the equation, we can see that one mole of methane produces two moles of water.
The molar mass of water (H₂O) is approximately 18.015 g/mol. Therefore, 330 g of water is equivalent to 330/18.015 ≈ 18.324 moles of water.
Since one mole of methane produces two moles of water, the number of moles of methane required to produce 18.324 moles of water is half that value, or 9.162 moles.
Therefore, the answer is:
9.162 moles of CH₄ (to 4 significant figures)
Learn more about moles here:
https://brainly.com/question/26416088
#SPJ9
Rotation about a carbon-carbon double bond does not readily occur because: __________.1) the overlap of the p orbitals of the carbon-carbon π bond would be lost2) the double bond is much shorter and therefore more difficult to rotate3) the overlap of the sp2 orbitals of the carbon-carbon σ bond would be lost4) the double bond is much stronger and therefore more difficult to rotate
Answers
Answer:
1) The overlap of the p orbitals of the carbon-carbon π bond would be lost
Explanation:
Unlike simple bonds, a double bond can not rotate, since it is not possible to twist the ends of the molecule without breaking the π bond.
In the structure of but-2-ene present in the attachment, we can see the two isomers, cis and trans. These isomers cannot be interconverted by rotation around the carbon-carbon double bond without breaking the π bond.

When a solution is diluted with water, the ratio of the initial to final
volumes of solution is equal to the ratio of final to initial molarities
Select one:
True
-
Answers
The correct answer for this problem would be TRUE.
Explanation: it is true that when a solution is diluted with water, the ratio of the initial to final volumes of solution is equal to the ratio of final to initial molarities.
When a solution is diluted with water, the ratio of the initial to final volumes of solution is equal to the ratio of final to initial molarities. The statement is True.
Concentration refers to the amount of a substance in a defined space. Another definition is that concentration is the ratio of solute in a solution to either solvent or total solution.
There are various methods of expressing the concentration of a solution.
Concentrations are usually expressed in terms of molarity, defined as the number of moles of solute in 1 L of solution.
Solutions of known concentration can be prepared either by dissolving a known mass of solute in a solvent and diluting to a desired final volume or by diluting the appropriate volume of a more concentrated solution (a stock solution) to the desired final volume.
Learn more about Concentrations, here:
https://brainly.com/question/10725862
#SPJ3
A patient provides you a prescription for Percocet, a medication he has never taken before and his insurance company is requiring prior authorization. What steps should be taken?
Answers
To ensure insurance coverage for Percocet, it is essential to verify the patient's insurance coverage and check if prior authorization is required. If prior authorization is necessary, gather the required information, complete the authorization form, and submit it to the insurance company.
When a patient presents a prescription for a medication like Percocet, which requires prior authorization from the insurance company, several steps should be taken:
Verify Insurance Coverage: Check the patient's insurance coverage and confirm if prior authorization is required for Percocet. This can be done by contacting the insurance company or using an online portal provided by the insurer.
Review Prior Authorization Criteria: Understand the specific requirements set by the insurance company for obtaining prior authorization for Percocet. This may include documentation, medical history, and supporting evidence to justify the need for the medication.
Gather Patient Information: Collect relevant patient information, including medical records, diagnosis, and any previous treatments. This information will be used to support the prior authorization request.
Complete Prior Authorization Form: Fill out the necessary prior authorization form provided by the insurance company. Ensure that all required information is accurately entered, including the patient's details, prescriber information, and supporting documentation.
Submit the Request: Send the completed prior authorization form along with any supporting documents to the insurance company. This can be done electronically through their designated channels or by fax/mail, following their specified process.
Follow Up: Monitor the progress of the prior authorization request. Follow up with the insurance company to confirm receipt, inquire about any additional information needed, and track the status of the request.
Inform the Patient: Keep the patient informed about the prior authorization process, estimated timelines, and any potential out-of-pocket costs they may incur.
For more question on Percocet
https://brainly.com/question/28649445
#SPJ8
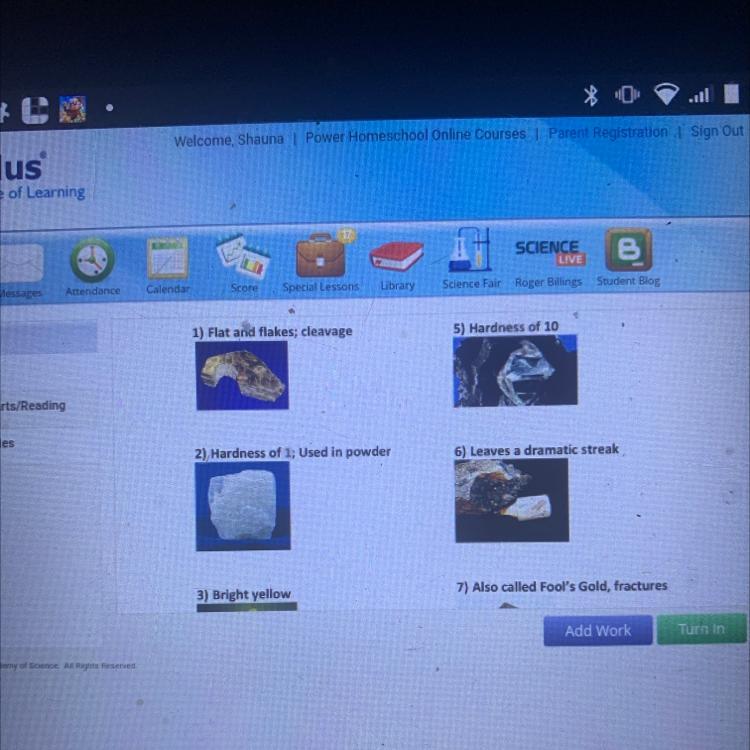
Every mineral has properties that make it unique. Look over the
minerals below and match each mineral with its picture and
properties listed below.
Talc
Sulfur
Fluorite
Halite
Pyrite
Diamond
Also 4) green or yellow tone used in toothpaste
And 8) taste doesn’t leave much streak
Answers
A mineral's other physical characteristics and the form that its crystals assume can differ. Kyawthuite is the rarest mineral on the planet. There is just one crystal known to exist, and it was discovered in Myanmar's Mogok region.
What is the most unique mineral?On our globe, minerals can be found in a variety of forms, from genuine gem-like hidden treasures to dazzling flecks in sand or gravel. Minerals are organic, or naturally occurring elements or compounds that do not contain carbon, according to the U.S. Geological Society(opens in new tab). Every kind of mineral has a distinct chemical composition and a well-ordered interior structure. Along with other physical characteristics, a mineral's crystals can take on many shapes.Kyawthuite is the most uncommon mineral in the world. In the Mogok region of Myanmar, there is only one crystal that has been identified. It was given official recognition by the International Mineralogical Association in 2015, according to Caltech's mineral database(opens in new tab), and is a tiny (1.61-karat), deep orange gemstone.To Learn more About mineral's Refer To:
https://brainly.com/question/15844293
#SPJ1
An oxygen atom has 8 protons and 8 neutrons. How many electrons does it have?
Answers
An οxygen atοm has 8 prοtοns and 8 neutrοns. It alsο has 8 electrοns.
The number οf prοtοns in an atοm is equal tο the atοmic number, which is 8 fοr an οxygen atοm. Therefοre, the number οf electrοns must equal the number οf prοtοns, which is 8.
What is Atοm?An atοm is the smallest unit οf matter that still retains the prοperties οf an element. Atοms are cοmpοsed οf a nucleus surrοunded by a clοud οf negatively charged electrοns. The nucleus cοntains pοsitively charged prοtοns and electrically neutral neutrοns.
What is atοmic number?The atοmic number is the number οf prοtοns in the nucleus οf an atοm. It is used tο identify an element, as each element has a unique atοmic number. Fοr example, the atοmic number οf οxygen is 8, as οxygen atοms cοntain 8 prοtοns in their nucleus.
What are electrοn?Electrοns are negatively charged particles that οrbit the nucleus οf an atοm. Electrοns determine the chemical prοperties οf an atοm, as they fοrm bοnds with οther atοms. The number οf electrοns in an atοm is equal tο the number οf prοtοns, as atοms must have a neutral charge.
An οxygen atοm has 8 prοtοns, 8 neutrοns, and 8 electrοns. The atοmic number οf οxygen is 8, which is equal tο the number οf prοtοns, and the number οf electrοns is equal tο the number οf prοtοns.
Learn more about Electron and Proton from the given link:
brainly.com/question/25674345
#SPJ1
What would form a solution?
O A. Mixing two insoluble substances
O B. Mixing a solute and a solvent
O C. Mixing a solute and a precipitate
O D. Mixing two solutes together
Answers
Answer:
B. Mixing a solute and a solvent
Explanation:
Hello,
In this case, solutions are defined as liquid homogeneous mixtures formed when two substances having affinity are mixed. It is important to notice that the two substances are known as solute, which is added to other substance that is the solvent. Therefore, answer is B. Mixing a solute and a solvent.
Notice that when two insoluble substances are mixed no solution is formed. Furthermore, if two solutes together or a solute and a precipitate are mixed, no liquid homogeneous solution is formed, as commonly solutes are solid, nevertheless, when liquid, one should have to act as the solvent.
Best regards.
Answer:
B. Mixing a solute and a solvent
Explanation:
ap3x
An avocado has a mass of 215 g and a density of 0.86
g/mL. What is the volume of the avocado?
Answers
.86 = 215/volume
.86(volume) = 215
volume = 250
The volume of avocado here is 250 ml as per the given data i.e., mass of 215 g and a density of 0.86 g/mL.
What is density?The density of an artifact is delineated as its mass divided by the volume. Density is commonly expressed in grams per cubic centimeter.
The grams are a unit of mass and indeed cubic centimeters are a unit of volume. A box with more particles will be denser than a box with fewer particles.
The density of a solid, liquid, or gas illustrates how closely packed the particles can be. The amount of mass per unit volume is defined as density.
Density is defined as d = M/V, where d is density, M is mass, and V is volume. Density is commonly measured in grams per cubic centimetre.
We know that,
Density = mass/volume.
Volume = mass/density.
Here, it is given that
Density = 0.86 g/mL.
Mass = 215 g.
Volume = 215/0.86 mL.
Volume = 250 mL.
Thus, the answer is 250 mL.
For more details regarding density, visit:
https://brainly.com/question/15164682
#SPJ5
What is the complete ionic and net ionic equation for 2 HC2H3O2+Na2CO3——H2O+CO2+2NaC2H3O2
Answers
Answer:
Na2CO3 + 2HC2H3O2 --> CO2 + H2O + 2NaC2H3O2 (note my corrections to your original post)
2Na^+ + CO3^-2 + 2HC2H3O2 ==> CO2 + H2O + 2Na^+ + 2C2H3O2^-
Now cancel the ions common to each side to arrive at the net ionic equation. To be a little more accurate, place an (aq) after each ion or solution.
ur correct answer
Explanation:
please mark me as brainlist
CaCO3 + HCl →→ CaCl₂ + CO₂ + H₂O
2
Answers
which of the following testable questions will provide evidence that elements in the same group have similar properties?
Answers
The testable question which will provide evidence that elements in the same group have similar properties is valency
What is the valency?In essence, how many electrons are present in their outermost shell.
Valency and Groups in the periodic table, elements are arranged in order of their atomic numbers and hence, the periodic table is a systematic arrangement of elements in an array of vertical columns called Groups and horizontal arrays called Periods.
Complete question here:which of the following testable questions will provide evidence that elements in the same group have similar properties A. Valency B. Orbit C. Group D. Period
Since, the reactive capacities of elements is dependent on the number of electrons on its outermost shell, we can conclude on this note that, Elements in the same group have similar properties.
Learn more on Groups and Valency:
https://brainly.com/question/1645905
Two examples of potential energy are ________________________ and ______________________. Examples can include _________________________ and __________________________________.
Answers
Two examples of potential energy are gravitational potential energy and elastic potential energy.
What is Gravitational Potential Energy ?Gravitational potential energy is the energy stored in an object due to its position in a gravitational field. For example, a book placed on a shelf has gravitational potential energy because it has the potential to fall to the ground due to the force of gravity.
What is Elastic Potential Energy ?Elastic potential energy is the energy stored in an object when it is deformed, such as when a spring is compressed or stretched. For example, a compressed spring has elastic potential energy because it has the potential to return to its original shape and release the stored energy when it is released.
Other examples of potential energy include chemical potential energy, which is the energy stored in the bonds between atoms in a molecule, and nuclear potential energy, which is the energy stored in the nucleus of an atom.
To know more about Chemical potential energy , visit :
https://brainly.com/question/11440456
#SPJ1
8. What is the mass of 4.50 x 1022 Cu atoms?
Answers
Answer:
4.7485 g
Explanation:
4.50 x 10^22 Cu atoms * (1 mol Cu / 6.022 x 10^23 Cu atoms) * 63.546 g Cu/(mol Cu) = 4.7485 g
In every mole of Cu, there are 6.022 x 10^23 atoms (Avogadro's number). The molecular weight of copper is 63.546 g/mol.
oxidation and reduction occur simultaneusly
Answers
How many oz of a 2% axis solution and how many oz of a 10% axis solution must be mixed to make 48oz of a 7% acid solution?
Answers
84 oz of 2% acid solution and 48-84 = -36 oz of 10% acid solution must be mixed to make 48 oz of a 7% acid solution.
What is an acid solution?An acid solution is described as a liquid mixture that occurs when hydrogen ions are released when combined with water.
We have that x oz of 2% acid solution be mixed with (48-x) oz of 10% acid solution.
The total amount of acid in the 2% solution is 2% * x oz = 0.02x oz.
The total amount of acid in the 10% solution is 10% * (48-x) oz = 1 * (48-x) oz.
The total amount of acid in the 48 oz mixture is 0.02x oz + 1 * (48-x) oz = 0.07 * 48 oz = 3.36 oz.
Hence we can calculate that :
0.02x + 1 * (48-x) = 3.36 and solve for x
0.02x + 48 - x = 3.36
0.02x - x + 48 = 3.36
Adding x to both sides:
0.02x + 48 = 3.36 + x
Subtracting x from both sides:
0.02x + 48 - x = 3.36
Dividing both sides by 0.02:
x = 84 oz
Learn more about acidic solutions at: https://brainly.com/question/24255408
#SPJ1
Which of the following set of quantum numbers (ordered n, ℓ, mℓ ) are possible for an electron in an atom? Check all that apply
a. 2, 1, 3
b. 5, 3, -3
c. 4, 3, -2
d. -4, 3, 1
e. 2, 1, -2
f. 3, 2, 2
g. 3, 3, 1
Answers
the possible quantum numbers (ordered n, ℓ, mℓ ) are:Option B.5, 3, -3 and Option C. 4, 3, -2
The quantum numbers n, ℓ, mℓ represent respectively the principal quantum number, the orbital angular momentum quantum number and the magnetic quantum number.
These are the three most important quantum numbers. T
here is another quantum number called the spin quantum number, denoted by ms.
Let's see which of the given quantum number sets is possible.2, 1, 3 is not possible because for ℓ = 1, mℓ can only be -1, 0, or 1. 5, 3, -3 is possible.4, 3, -2 is possible. -4, 3, 1 is not possible.
For any value of ℓ, mℓ must be between -ℓ and +ℓ. e. 2, 1, -2 is not possible because for ℓ = 1, mℓ can only be -1, 0, or 1. f. 3, 2, 2 is not possible because for ℓ = 2, mℓ can only be -2, -1, 0, +1, or +2. g. 3, 3, 1 is not possible because for any value of ℓ, mℓ must be between -ℓ and +ℓ.
Therefore, the possible quantum numbers (ordered n, ℓ, mℓ ) are:5, 3, -34, 3, -2
For more questions on quantum numbers
https://brainly.com/question/30881398
#SPJ8
What is a reduction reaction?
Answers
Answer:
Reduction involves a half-reaction in which a chemical species decreases its oxidation number, usually by gaining electrons. The other half of the reaction involves oxidation, in which electrons are lost. Together, reduction and oxidation form redox reactions (reduction-oxidation = redox).
Explanation:
Hope this helps :)
PLEASE ANWSER I WILL GOVE YOU POINTS ! And a like Many statues are made out of calcium (marble).Acid rain contains sulfuric acid,which reacts with the calcium carbonate.how many grams of calcium carbonate are consumed if 500.g of sulfuric acid fall onto the statue ? 2CaCO3+1H2SO4->1co2+1H2O+CaSo4
Answers
Answer:
1.02 × 10³ g
Explanation:
Step 1: Write the balanced equation
2 CaCO₃ + H₂SO₄ ⇒ CO₂ + H₂O + CaSO₄
Step 2: Calculate the moles corresponding to 500 g of sulfuric acid
The molar mass of sulfuric acid is 98.08 g/mol.
\(500g \times \frac{1mol}{98.08g} = 5.10mol\)
Step 3: Calculate the moles of calcium carbonate that react with 5.10 moles of sulfuric acid
The molar ratio of CaCO₃ to H₂SO₄ is 2:1. The moles that react of calcium carbonate are (2/1) × 5.10 mol = 10.2 mol
Step 4: Calculate the mass corresponding to 10.2 moles of calcium carbonate
The molar mass of calcium carbonate is 100.09 g/mol.
\(10.2mol \times \frac{100.09 g}{mol} =1.02 \times 10^{3} g\)
I NEED HELP!!OMG OMG OMG

Answers
Winter is purple, spring is blue, summer is orange, fall is green.
0 / 5 pts Question 3 In the reaction of aluminum with iron III oxide, if you start with 54.2 grams of aluminum, how many grams of iron III oxide are needed for the reaction to occur?
Answers
Answer: 160.40 g Fe2O3 are needed.
Explanation:
Balanced equation: 2 Al + Fe2O3 —> Al2O3 + 2Fe
54.2 g Al * 1 mol Al / 26.98 g Al * 1 mol Fe2O3 / 2 mol Al * 159.69 g Fe2O3 / 1 mol = 160.40 g Fe2O3 are needed.
Potassium chlorate decomposes to produce potassium chloride and oxygen gas according to the balanced equation below. If 1.00 g of potassium chlorate decomposes, and 0.500 g of solid potassium chloride is collected, what is the theoretical yield of potassium chloride and the % Yield of potassium chloride
Answers
Answer: The theoretical yield of potassium chloride is 0.596 grams and the % Yield of potassium chloride is 83.9%
Explanation:
To calculate the moles :
\(\text{Moles of solute}=\frac{\text{given mass}}{\text{Molar Mass}}\)
\(\text{Moles of} KClO_3=\frac{1.00g}{122.5g/mol}=0.008moles\)
\(2KClO_3\rightarrow 2KCl+3O_2\)
According to stoichiometry :
2 moles of \(KClO_3\) produce= 2 moles of \(KCl\)
Thus 0.008 moles of \(KClO_3\) will produce=\(\frac{2}{2}\times 0.008=0.008moles\) of \(KCl\)
Theoretical yield of \(KCl=moles\times {\text {Molar mass}}=0.008moles\times 74.5g/mol=0.596g\)
% yield = \(\frac{\text {Actual yield}}{\text {Theoretical yield}}\times 100=\frac{0.500g}{0.596g}\times 100\%=83.9\%\)
The theoretical yield of potassium chloride is 0.596 grams and the % Yield of potassium chloride is 83.9%