The equation for Plutonium (Pu) reaction is: (attachment)
Please complete the symbol for the plutonium nucleus produced.
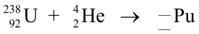
Answers
The complete nuclear fusion reaction for the Plutonium produced is \(^{238}_{92}U \ + \ ^{4}_{2}He \ \ --- > \ _{94}^{242}P\).
What is nuclear fusion?Nuclear fusion is a reaction in which two or more atomic nuclei are combined to form one or more different atomic nuclei and subatomic particles.
The complete fusion reaction of Uranium to form Plutonium;
\(^{238}_{92}U \ + \ ^{4}_{2}He \ \ --- > \ _{94}^{242}P\)
Thus, the complete nuclear fusion reaction for the Plutonium produced is \(^{238}_{92}U \ + \ ^{4}_{2}He \ \ --- > \ _{94}^{242}P\).
Learn more about nuclear fusion here: https://brainly.com/question/1349098
#SPJ1
Related Questions
Number 2 & 3 please !!<3

Answers
Answer:just for the ponits
Explanation:ima be a jenna
Choose all the answers that apply.
Argon (Ar) is in group 18. Argon ____.
1. is part of the noble gas group
2. has 18 electron shells
3. does not react readily with other elements
4. has properties similar to other elements in group 18
5. forms a salt when combined with a metal
Answers
Answer:
answer is 2 I think I'm correct sorry if I'm not
A container of gas is initially at 0.25 atm and 0 ˚C. What will the pressure be at 125 ˚C?
Answers
Answer:
0.37atm
Explanation:
Given parameters:
Initial pressure = 0.25atm
Initial temperature = 0°C = 273K
Final temperature = 125°C = 125 + 273 = 398K
Unknown:
Final pressure = ?
Solution:
To solve this problem, we use a derivative of the combined gas law;
\(\frac{P1}{T1}\) = \(\frac{P2}{T2}\)
P and T are pressure and temperature
1 and 2 are initial and final values
\(\frac{0.25}{273}\) = \(\frac{P2}{398}\)
P2 = 0.37atm
write the line formular,condensed formular and structural formular of 3-ethyl-1,2-dimethylheptane
Answers
This formula is wrong because a substituent can't attach with 1st carbon in chain or the here the no. of carbon in carbon chain is 8
A sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy of combustion of graphite. The temperature of the calorimeter increases from 26.74 °C to 27.93 °C. What is the heat capacity of the calorimeter and its contents?
Answers
Answer:
The correct answer is 15.54 kJ per degree C.
Explanation:
The enthalpy change for one mole of a substance, which combines or burns with the oxygen under the standard conditions, that is, at 25 degree C and 1 bar pressure is known as the standard molar enthalpy of combustion. The amount of heat transferred can be calculated by using the formula, q = mcΔT -------------(i)
Here q is the amount of heat transferred, c is the specific heat, ΔT is the change in temperature, and m is the mass of the substance. As in case of bomb calorimeter, mass if considered constant, thus, for calorimeter the equation mentioned will become, q = cΔT ---- (ii)
The standard molar enthalpy of combustion for carbon is -393.5 kJ/mol, that is, -393.5 kJ per mole of heat is generated by burning one mole of carbon. The molecular mass of carbon is 12 gram per mole.
Thus, the number of moles of carbon equivalent to 0.562 grams of carbon can be determined as,
Number of moles of carbon = mass / molecular mas
= 0.562 grams / 12 gram per mole
= 0.047 mol
The heat generated by burning 0.562 grams or 0.047 mole will be,
q = ΔH° × number of moles
= (-393.51 kJ/mol) × 0.047 mol
= -18.49 kJ, the negative sign shows that the heat is produced.
To find heat capacity of calorimeter, put the value of q as -18.49 kJ, for ΔT as (27.93 °C - 26.74 °C) in the equation (ii)
18.49 kJ = c × (27.93 - 26.74)
c = 18.49 kJ/1.19 °C
c = 15.54 kJ/°C
How are minerals identified?
Answers
Which star has the highest surface temperature
A. blue star
B. yellow star
C. white star
D. red star
Answers
Answer:
The blue star is the hottest star.
Explanation:
Red stars are cooler than the sun, with surface temperatures of 3,500 K for a bright red star and 2,500 K for a dark red star. The hottest stars are blue, with their surface temperatures falling anywhere between 10,000 K and 50,000 K
what demonstrated Kinetic Energy
Answers
Kinetic Energy Statement
Kinetic energy is energy that a body possess as a result of its motion. Kinetic energy as it is mathematically written is the "classic statement" of: Kinetic energy is equal to half the mass of an object times its velocity squared.
There are five types of kinetic energy: radiant, thermal, sound, electrical and mechanical. Let us look at some of the kinetic energy examples and learn more about the different types of kinetic energy.
Hope this helped!
❤️
Long Life Floors is expected to pay an annual dividend of $4 a share and plans on increasing future dividends by 3 percent annually. The discount rate is 14 percent. What will the value of this stock be 5 years from today (in $ dollars)
Answers
The value of Long Life Floors stock 5 years from today will be approximately $27.67 per share.
To calculate the value of the stock, we can use the dividend discount model (DDM). The DDM takes into account the present value of expected future dividends. In this case, the expected future dividends can be calculated by increasing the current dividend of $4 by 3 percent annually for five years. So, the expected dividends in five years would be $4 * (1 + 0.03)^5 = $4.62 per share.
Using a discount rate of 14 percent, we can calculate the present value of the expected future dividends. The formula for calculating the present value is: Present Value = Future Value / (1 + Discount Rate)^n, where n is the number of periods (in this case, 5 years).
Using the formula, the present value of the expected future dividends is: $4.62 / (1 + 0.14)^5 = $2.376 per share.
Therefore, the value of Long Life Floors stock 5 years from today is approximately $2.376 per share.
learn more about DDM here
brainly.com/question/32132762
#SPJ11
Which is a mixture?
Sodium metal
chlonne gas
sodium metal and chlonne gas
sodium chlonde (salt) and water
Answers
Answer:
I believe it's salt and water
Explanation:
not sure
Answer:
Mixture is a electronic device used to peel the things such as onion ,tomato,ginger, garlic etc.
Mark the statements which are correct. (Select all that apply. )
1 g = 10^3 mg
10^-3 g = 10^12 ng
1 s = 10^6 μs
1 km = 10^5 mm
1 s = 10^3 ms
Answers
All statements given in the question are incorrect except for 1 statement. The correct statement is:1 s = 10^3 ms.
In the question, we have been provided with 5 statements. We are asked to select all the correct statements from those 5 statements. Given below are for each statement:1 g = 10^3 mg:This is incorrect. 1 g is equal to 1000 mg.10^-3 g = 10^12 ng:This is incorrect. 10^-3 g is equal to 1 mg.1 km = 10^5 mm:This is incorrect. 1 km is equal to 1,000,000 mm.1 s = 10^6 μs:This is incorrect. 1 s is equal to 1,000,000 μs.1 s = 10^3 ms:This is correct. 1 s is equal to 1000 ms.Therefore, the main answer to this question is that only 1 statement is correct, which is:1 s = 10^3 ms.
Metric units are based on the power of ten. The base units of the International System of Units (SI) are the meter, kilogram, second, kelvin, ampere, mole, and candela. All other metric units can be derived from these basic units.The first unit in each conversion is in grams, seconds, or kilometers. The metric units for millimeters, microseconds, and nanograms are derived from these basic units. One gram is equal to 1000 milligrams (mg), 1 second is equal to 1000 milliseconds (ms), and 1 kilometer is equal to 1000000 millimeters (mm). 10^-3 g is equal to 1 milligram (mg), 10^6 μs is equal to 1 second (s), and 10^12 ng is equal to 1 gram (g).
To know more about except visit:
https://brainly.com/question/14400269
#SPJ11
A student wants to determine the effect of a fertilizer on the growth of bean plants. She sets
up the following experiment.
Bean
Soil
Fertilizer
Daily water 50 mL
A
Bean
Soil
Daily water 50 mL
2. What does setup "B" represent and why is it important in this experiment?
ANSWER QUESTION 2 NOT QUESTION 1

Answers
The experimental set up in B is to observe the growth the green beans without the addition of fertilizer. Thus, we can compare the growth of the plant with and without fertilizer and thereby we can understand the effect the fertilizer on the plant growth.
What is fertilizer?Fertilizers are chemical substances used to nourish the plants by making the soil rich in nutrients and minerals. Plants needs minerals along with water such as potassium, calcium, phosphorous, nitrogen etc.
Sometimes the soil comes deficient of these minerals and we have to fertilize the soli by the addition of the chemicals containing these minerals. Hence, fertilizers provide a nutrient rich soil for plant growth.
The experimental set up with fertilizer added plant and the second one (B) without fertilizer helps to compare the growth in both conditions and we can clearly understand how fertilizers effect the growth of plants by comparing the plant growth in A and B.
To find more on fertilizers, refer here:
https://brainly.com/question/24196345
#SPJ1
20cm of 0.09M solution of H2SO4. requires 30cm of NaOH for complete neutralization. Calculate the
molar concentration of NaOH. (A) 0.12M (B) 0.0018M (C) 0.012M (D) 0.0036M
Answers
Answer:
Choice A: approximately \(0.12\; \rm M\).
Explanation:
Note that the unit of concentration, \(\rm M\), typically refers to moles per liter (that is: \(1\; \rm M = 1\; \rm mol\cdot L^{-1}\).)
On the other hand, the volume of the two solutions in this question are apparently given in \(\rm cm^3\), which is the same as \(\rm mL\) (that is: \(1\; \rm cm^{3} = 1\; \rm mL\).) Convert the unit of volume to liters:
\(V(\mathrm{H_2SO_4}) = 20\; \rm cm^{3} = 20 \times 10^{-3}\; \rm L = 0.02\; \rm L\).\(V(\mathrm{NaOH}) = 30\; \rm cm^{3} = 30 \times 10^{-3}\; \rm L = 0.03\; \rm L\).Calculate the number of moles of \(\rm H_2SO_4\) formula units in that \(0.02\; \rm L\) of the \(0.09\; \rm M\) solution:
\(\begin{aligned}n(\mathrm{H_2SO_4}) &= c(\mathrm{H_2SO_4}) \cdot V(\mathrm{H_2SO_4})\\ &= 0.02 \; \rm L \times 0.09 \; \rm mol\cdot L^{-1} = 0.0018\; \rm mol \end{aligned}\).
Note that \(\rm H_2SO_4\) (sulfuric acid) is a diprotic acid. When one mole of \(\rm H_2SO_4\) completely dissolves in water, two moles of \(\rm H^{+}\) ions will be released.
On the other hand, \(\rm NaOH\) (sodium hydroxide) is a monoprotic base. When one mole of \(\rm NaOH\) formula units completely dissolve in water, only one mole of \(\rm OH^{-}\) ions will be released.
\(\rm H^{+}\) ions and \(\rm OH^{-}\) ions neutralize each other at a one-to-one ratio. Therefore, when one mole of the diprotic acid \(\rm H_2SO_4\) dissolves in water completely, it will take two moles of \(\rm OH^{-}\) to neutralize that two moles of \(\rm H^{+}\) produced. On the other hand, two moles formula units of the monoprotic base \(\rm NaOH\) will be required to produce that two moles of \(\rm OH^{-}\). Therefore, \(\rm NaOH\) and \(\rm H_2SO_4\) formula units would neutralize each other at a two-to-one ratio.
\(\rm H_2SO_4 + 2\; NaOH \to Na_2SO_4 + 2\; H_2O\).
\(\displaystyle \frac{n(\mathrm{NaOH})}{n(\mathrm{H_2SO_4})} = \frac{2}{1} = 2\).
Previous calculations show that \(0.0018\; \rm mol\) of \(\rm H_2SO_4\) was produced. Calculate the number of moles of \(\rm NaOH\) formula units required to neutralize that
\(\begin{aligned}n(\mathrm{NaOH}) &= \frac{n(\mathrm{NaOH})}{n(\mathrm{H_2SO_4})}\cdot n(\mathrm{H_2SO_4}) \\&= 2 \times 0.0018\; \rm mol = 0.0036\; \rm mol\end{aligned}\).
Calculate the concentration of a \(0.03\; \rm L\) solution that contains exactly \(0.0036\; \rm mol\) of \(\rm NaOH\) formula units:
\(\begin{aligned}c(\mathrm{NaOH}) &= \frac{n(\mathrm{NaOH})}{V(\mathrm{NaOH})} = \frac{0.0036\; \rm mol}{0.03\; \rm L} = 0.12\; \rm mol \cdot L^{-1}\end{aligned}\).
why is OH on the outside of the lewis structure for methanol?
Answers
In the Lewis structure of methanol (CH3OH), the OH group is placed on the outside because it is an important functional group that influences the chemical properties and reactivity of the molecule.
The Lewis structure is a representation of a molecule that shows the arrangement of atoms and valence electrons. In methanol, carbon (C) is the central atom bonded to three hydrogen (H) atoms and one oxygen (O) atom. The oxygen atom forms a single bond with carbon and also has two lone pairs of electrons.
The placement of the OH group (hydroxyl group) on the outside of the Lewis structure is significant because it determines the chemical behavior of methanol. The OH group consists of an oxygen atom bonded to a hydrogen atom and represents the presence of an alcohol functional group.
In organic chemistry, functional groups are specific arrangements of atoms within a molecule that give rise to characteristic chemical reactions and properties. The presence and position of functional groups can greatly influence the behavior and reactivity of a compound. In the case of methanol, the hydroxyl group provides the molecule with its characteristic properties.
know more about valence electrons here:
https://brainly.com/question/371590
#SPJ8
Describe two types of nuclear reactions other than radioactive decay
Answers
Nuclear fusion: this is the joining of two small atomic nuclei into one nucleus. Nuclear fission: this is the splitting of one large atomic nucleus into smaller fragments.
After the removal of carbon, the oxygen in co2 ends up.
a. true
b. false
Answers
The given statement, After the removal of carbon, the oxygen in CO₂ ends up is False.
Carbon dioxide (CO₂) is a compound made up of two elements, carbon and oxygen, in a ratio of one carbon atom for every two oxygen atoms. When carbon dioxide is removed, the oxygen atoms do not remain isolated - instead, they bond with other oxygen atoms from the surrounding environment, forming oxygen gas (O₂).
Oxygen gas is highly reactive and forms strong bonds with other oxygen atoms to form molecules of the natural gas O₂. The result is that the oxygen that was part of the carbon dioxide is no longer present - it has become part of the newly formed oxygen gas molecules.
Oxygen gas is present in the atmosphere and it is highly reactive and mobile, meaning that it can quickly move and form bonds with other elements. When carbon dioxide is removed, the oxygen atoms that were part of the molecule become part of oxygen gas instead, creating molecules of the natural gas O₂.
know more about environment here
https://brainly.com/question/5511643#
#SPJ11
what kind of noncovalent interaction is typified by interactions between two molecules that are so close together that they can experience weak attractive forces bonding them together?
Answers
Van der Waals forces. Van der Waals forces are very weak forces between two very close surfaces.
Forces of van der Waals. When two surfaces are very close to one another, van der Waals forces are very weak.
Is the Van der Waals weak?In gases, liquefied and solidified gases, and nearly all organic liquids and solids, neutral molecules are drawn to one another by van der Waals forces, which are relatively weak electric forces.
Van der Waals interactions are nonionic forces that have a comparatively low energy of 0.5 to 1 kcal/mol.
The covalent link from its less electronegative Neighbour atom tends to drive the electron cloud toward neutral molecules with electronegative atoms like oxygen and nitrogen.
To know more about van der Waals forces visit:
https://brainly.com/question/13201335
#SPJ4
Use the molarity from Question 5 (0.86 M) to calculate the mass of acetic acid in 1.00 L of the vinegar solution.
Answers
The molarity unit of concentration is used to calculate the number of moles of a solute per liter of solution.
Therefore, The process for resolving molarity issues is rather straightforward. Here is a quick way to determine the molarity of a solution.
The key to calculating molarity is to keep in mind that it is measured in moles per liter (M).The number of moles of a solute dissolved in a liter of a solution is used to express a solute's molarity.
The density of acetic acid is 1.05 g/ml, and its molecular weight is 60 g/mol. Acetic acid, CH3COOH (C2H4O2), has a molecular weight of 60 grams. One liter of water and one mole of acetic acid combined to form a molar solution.
Thus, The number of moles of a solute per liter of solution is measured using the unit of concentration known as molarity.
Learn more about Molarity, refer to the link:
https://brainly.com/question/31545539
#SPJ1
Nucleus Nucleus Atom 1 Atorn 2 Nucleus Nucleus Atom 3 Atom 4 Which two atoms are of elements in the same group in the periodic table?
Answers
Explanation:
The atoms of elements on the same period on the periodic table typically have the same number of valence electrons.
They have the same electronic configuration.
Valence electrons are electrons in the outermost shell of an atom.
The number of electrons in an atom is a good indicator of elements that might potentially belong to the same group.
What is the pOH of water?

Answers
Answer:
A. 7
(assuming the water is neutral)
what happen in the house
Answers
Answer:
The film stars Will Ferrell, Amy Poehler, Jason Mantzoukas, Ryan Simpkins, Nick Kroll, Allison Tolman, Rob Huebel, Michaela Watkins, and Jeremy Renner, and follows a couple who open an underground casino in their friend's house in order to pay for their daughter's college tuition.
Explanation:
is this what ur talking about ?
Zirconium (Zr) has an average atomic mass of 91. 22 amu and is made up of the isotopes 90Zr, 91Zr, 92Zr, 94Zr, and 96Zr. The atom of which isotope has the greatest mass?
Answers
Zirconium (Zr) has an average atomic mass of 91.22 amu and is made up of the isotopes 90Zr, 91Zr, 92Zr, 94Zr, and 96Zr. The atom of which isotope has the greatest mass?To determine the isotope with the largest mass, we must first understand what isotopes are. Isotopes are atoms that have the same atomic number but a different number of neutrons, resulting in a different atomic mass.
As a result, we can determine the mass of a specific isotope by determining the number of neutrons it contains. This is done by subtracting the atomic number from the atomic mass.For example, in the case of 90Zr, the atomic number of zirconium is 40, and the atomic mass of this isotope is 90. As a result, the number of neutrons in this isotope is equal to 90 - 40 = 50. We can repeat this process for the other zirconium isotopes, as follows:
- For 91Zr, neutrons = 91 - 40 = 51
- For 92Zr, neutrons = 92 - 40 = 52
- For 94Zr, neutrons = 94 - 40 = 54
- For 96Zr, neutrons = 96 - 40 = 56
As a result, we can see that the isotope with the largest mass is 96Zr, with a mass of 96 atomic mass units.
Therefore, we can conclude that the atom of the isotope 96Zr has the greatest mass among all the isotopes of zirconium.
For such more question on atomic mass
https://brainly.com/question/30390726
#SPJ8
Zirconium (Zr) has an average atomic mass of 91. 22 amu and is made up of the isotopes 90Zr, 91Zr, 92Zr, 94Zr, and 96Zr. The atom of which isotope has the greatest mass is 96Zr.
What are isotopes?Isotopes are atoms of a single element with differing numbers of neutrons in their nuclei. In addition, isotopes have the same atomic number and, as a result, the same number of electrons, but different atomic masses or mass numbers due to their differing numbers of neutrons.Isotope abundances are different in different materials and can also be modified over time by radioactive decay or other processes.The mass of an atom is primarily determined by the number of neutrons and protons in its nucleus. Because the number of electrons in the atom's outermost shell determines its chemical behavior, the number of neutrons in an atom's nucleus has little impact on its chemical behavior.
Zirconium (Zr) has an average atomic mass of 91.22 amu and is made up of the isotopes 90Zr, 91Zr, 92Zr, 94Zr, and 96Zr. To determine which of these isotopes has the greatest mass, look at the atomic number of each isotope:90Zr has a mass of 89.904 amu91Zr has a mass of 90.904 amu92Zr has a mass of 91.905 amu94Zr has a mass of 93.906 amu96Zr has a mass of 95.908 amuThe atom with the highest mass is 96Zr, which has a mass of 95.908 amu. Therefore, the atom of which isotope has the greatest mass is 96Zr.
To know more about Zirconium visit:-
https://brainly.com/question/28488357
#SPJ11
Cobalt is one of many metals that can be oxidized by nitric acid. Balance the following the reaction in acidic conditions. How many electrons are transferred, and what would be the coefficient for H_2 O in the net balanced reaction? Co(s) + HNO_3 (aq) rightarrow NO (aq) + Co^+2 (aq)
O A) 3 electrons; 2 H_2
O B) 2 electrons; 2 H_2
O C) 6 electrons; 6 H_2
O D) 2 electrons. 4 H_2
O E) 4 electrons; 2 H_2 O
Answers
The balanced equation for the reaction in acidic conditions is:
3Co(s) + 8HNO3(aq) → 3NO(g) + 2H2O(l) + 3Co^2+(aq). Hence the answer is option C) 6 electrons; 6 H2O.
In this reaction, 6 electrons are transferred. The coefficient for H2O in the net balanced reaction is 2. To balance the equation, we start by balancing the atoms other than hydrogen and oxygen. We can see that the cobalt (Co) atoms are already balanced, and on the left side, we have 3 nitrogen (N) atoms and 8 hydrogen (H) atoms from the nitric acid (HNO3). On the right side, we have 3 nitrogen (N) atoms and 6 hydrogen (H) atoms from the NO and H2O.
Next, we balance the hydrogen atoms by adding 2 water (H2O) molecules on the right side. This gives us a total of 8 hydrogen (H) atoms on both sides. Now, we have 3 nitrogen (N) atoms on the left and 3 nitrogen (N) atoms on the right.
Finally, we balance the charge by adding 6 electrons (e-) to the left side, as the cobalt (Co) is oxidized from Co(s) to Co^2+(aq). This accounts for the transfer of 6 electrons in the reaction.
6 electrons are transferred in the reaction, and the coefficient for H2O in the net balanced reaction is 2. Therefore, the correct answer is C) 6 electrons; 6 H2O.
Learn more about balanced equation here: brainly.com/question/31242898
#SPJ11
S2O3 2- + OH- → SO4 2- + H2O + e-. After the above half-reaction is balanced, which of the following are the respective coefficients of OHand SO4 2-? (A) 8 and 3 (B) 6 and 2 (C) 10 and 2 (D) 5 and 2 (E) 5 and 1
Answers
The balanced half-reaction is \(S_2O_3 ^2- + 3OH^- -- > SO_4^ {2-} + H_2O + e^-\). The respective coefficients of OH- and \(SO_4^ 2-\) are 3 and 1, so the correct answer is (E) 5 and 1.
To balance the given half-reaction, \(S_2O_3^2- + OH^- -- > SO_4^ 2- + H_2O + e^-\), we need to make sure that the number of atoms and charges are equal on both sides. Let’s examine the reaction and determine the coefficients of OH- and \(SO_4^ 2-\).
On the reactant side, we have \(S_2O_3 ^2-\) and OH-. The \(S_2O_3 ^2-\)- ion has a total charge of 2-. To balance the charge, we need 2 OH- ions with a charge of 1- each. So far, the equation looks like this:
\(S_2O_3^2- + OH^- -- > SO_4^ 2- + H_2O + e^-\)
Next, let’s balance the atoms. We have 3 oxygen atoms on the reactant side and 4 oxygen atoms on the product side . To balance the oxygen, we need to add 1 more OH- ion:
\(S_2O_3 ^2- + 3OH^- -- > SO_4^ {2-} + H_2O + e^-\)
Now, the equation is balanced with respect to charge and oxygen. The respective coefficients of OH- and \(SO_4^ 2-\) are 3 and 1, so the correct answer is € 5 and 1.
Therefore, the balanced half-reaction is \(S_2O_3 ^2- + 3OH^- -- > SO_4^ {2-} + H_2O + e^-\)
Learn more about half-reaction here:
https://brainly.com/question/18403544
#SPJ11
can any machine be ideal
Answers
It is not possible to construct an ideal machine. Because machines have some loss of energy in the form of heat or friction.
Contrast the three types of intermolecular forces.
Answers
Answer: The three major types of intermolecular interactions are dipole–dipole interactions, London dispersion forces (these two are often referred to collectively as van der Waals forces), and hydrogen bonds.
Hope this helps............ Stay safe and have a Merry Christmas!!!!!!! :D
The final electron acceptor in aerobic respiration is....... NAD water oxygen pyruvate O hydrogen . Answer al Question 16 Which of the following processes generate carbon dioxide? Hint There are more than one. Glycolysis Oxidative Phosphorylation The Link Reaction (pyruvate oxidation) The Citric Arid Cycle Lactic Acid Fermentation Alcoholic Fermentation
Answers
The final electron acceptor in aerobic respiration is oxygen (O2).The electron transport chain (ETC) in cellular respiration relies on a final electron acceptor to help oxygen get reduced into water. This is why oxygen is considered the final electron acceptor in cellular respiration.
During cellular respiration, glucose is broken down into pyruvate. Pyruvate is then transformed into acetyl CoA and enters the citric acid cycle, where it is oxidized and generates ATP, NADH, and FADH2. The final stage of aerobic respiration involves the electron transport chain, in which electrons from NADH and FADH2 are passed through a series of proteins and coenzymes in the inner mitochondrial membrane, ultimately reducing oxygen to form water.
This process is known as oxidative phosphorylation.In conclusion, the final electron acceptor in aerobic respiration is oxygen (O2), and carbon dioxide is generated in the link reaction (pyruvate oxidation) and the citric acid cycle.
To know more about cellular respiration visit:-
https://brainly.com/question/32872970
#SPJ11
Why can cows digest cellulose, while humans cannot?
pls dont go og le
Answers
Explanation:
Cause, Cow possess enzyme cellulase......which digests cellulose......and man doesn't possess such an enzyme...that's why man can't digest cellulose
Here it's....!
It's the solution of math question...
Hope it helps....☺☺

a cream contains 20 g of active ingredient in 60 g of cream base. what is the ratio strength?
Answers
When a cream includes 20 g of an active ingredient and 60 g of a cream base, the strength is 20:60.
A ratio with the form 1 in r serves as an expression for ratio strength. The comparable fraction's numerator would be 1, making it. According to accepted practice, the weight and volume are stated in grams and milliliters, respectively, when ratio strength denotes a solid in a liquid using units of weight and volume.
Ingredients are the materials required to create anything, particularly all the many foods you use to prepare a certain dish. Add the remaining components and combine. Countable noun One of a situation's key components is an ingredient. Any component that is used in a food to produce a certain result is considered an ingredient. Food additives are considered food ingredients.
Learn more about Ingredient here:
https://brainly.com/question/30141107
#SPJ4
The complete question is:
A cream contains 20 g of the active ingredient in 60 g of cream base. What is the ratio strength?
Select one:
2: 60
20: 60
200: 60
200: 600
ANSWER QUICK PLEASE!!!! 25 POINTS
Acrylonitrile and ethyl acetate have the same boiling point: 77.2ºC. In one to two sentences, explain what outcome you would expect if a mixture of these two substances were distilled.
Answers
The two substances can never be separated by distillation because distillation depends on difference in boiling point.
Distillation is a separation method that depends on difference in boiling point between two substances.
Usually, the substance having a lower boiling point is collected first as the temperature is gradually raised. The substance having a higher boiling point then follows.
However, since the two substances have the same boiling point, they can not be separated by distillation because they will be converted to vapor simultaneously thereby making separation impossible.
Learn more: https://brainly.com/question/15946045