the critical pressure of a substance is the a. pressure at which liquid and vapor phases are in equilibrium at 1 atmosphere b. lowest pressure above which a substance cannot be liquified at any temperature c. pressure at which the solid, liquid, and vapor phases are all in equilibrium d. pressure at which the vapor pressure of the liquid is equal to the external pressure e. pressure at which the vapor pressure of the liquid is equal to 760 mmhg
Answers
Option (b) is correct. The lowest pressure above which a substance cannot be liquified at any temperature. is the critical pressure.
The Critical pressure is defines as the vapor pressure of a fluid at the critical temperature above which distinct liquid and gas phases do not exist. As the critical temperature is approached, the properties of the gas and liquid phases become the same, resulting in only one phase. The point at which the critical temperature and critical pressure is met is called the critical point. It can also exist in equilibrium with a liquid (or solid), in which case the gas pressure equals the vapor pressure of the liquid or solid. The minimum pressure needed to liquefy it at the critical temperature. is the critical pressure.
To learn more about Critical pressure please visit:
https://brainly.com/question/1476460
#SPJ4
Related Questions
Base your answer to the following question on the information below and on your knowledge of
chemistry.
Wood is mainly cellulose, a polymer produced by plants. One use of wood is as a fuel in
campfires, fireplaces, and wood furnaces. The molecules of cellulose are long chains of repeating
units. Each unit of the chain can be represented as CHI1oOs. The balanced equation below
represents a reaction that occurs when CoH1oOs is burned in air.
C6H1100, + 602 + 6C02 + 5H120 + heat
Show a numerical setup for calculating the percent composition by mass of carbon in C6H100s
(gram-formula mass = 162. 1 g/mol)
Answers
To calculate the percent composition by mass of carbon in \(C_6H_{100}\), we need to determine the molar mass of carbon in the compound and divide it by the molar mass of the entire compound.
The balanced equation given indicates that for every 1 mole of \(C_6H_{100}\) burned, 6 moles of \(CO_2\) are produced. Using the molar masses of carbon and \(C_6H_{100}\) , we can calculate the percent composition by mass of carbon.
The molar mass of carbon (C) is 12.01 g/mol. The molar mass of \(C_6H_{100}\) can be calculated as follows:
(6 × 12.01 g/mol) + (1 × 1.01 g/mol) + (10 × 16.00 g/mol) = 162.10 g/mol.
To determine the percent composition by mass of carbon in \(C_6H_{100}\) , we need to find the mass of carbon in 1 mole of \(C_6H_{100}\) . Since there are 6 carbon atoms in the compound, the mass of carbon is:
(6 × 12.01 g/mol) = 72.06 g.
Now we can calculate the percent composition by mass of carbon:
(72.06 g / 162.10 g) × 100% ≈ 44.5%.
Therefore, the percent composition by mass of carbon in \(C_6H_{100}\) is approximately 44.5%.
To learn more about molar mass refer:
https://brainly.com/question/837939
#SPJ11
The sentences below are things people might say if they were planning to invest or not planning to invest Sort them into the correct categories . am thinking about buying stocks Planning to Invest Not Planning to Invest don't know much about investing can't afford to buy stocks don't really like to take risks need a way to manage my money want to save money for my future
Answers
Investing :
"I'm considering investing in equities."
"I need a system for handling my finances."
"I need to put money away for the future."
No Investment :
"My knowledge of investment is limited."
"I am unable to purchase stocks."
"I don't particularly enjoy taking chances."
Explanation:
These are accurate because I recently completed the course that taught me these things.
Learn ore about investing and no investment from here :
https://brainly.com/question/4459036?referrer=searchResults
#SPJ10
what are the rules for calculating protons
Answers
Answer:
Find the element,
Explanation:
Finding the element will tell you how many protons there are. For example, if you are given an atom of oxygen, you can figure out the number of protons by looking it up on a periodic table, which gives you 8.
If an atom is neutral, find the number of electrons. This number will also be the number of protons.
Help help help pleasee
17. (04.03 mc)
describe the characteristics of a double replacement reaction, how it is identified, and types of compounds
in the reactants and products. give an example in your response. (10 points)
Answers
The Double displacement reaction is the reaction in which the reacting substances react and exchange the ions and forms the new product.
The The Double displacement reaction is the reaction in which the reacting substances react and exchange the ions and forms the new product. The positive ions exchange the negative ions. The general examples is given as :
A⁺B⁻ + C⁺D⁻ ----> A⁺D⁻ + C⁺B⁻
In the double displacement reaction one of the product is :
The precipitate , The water or A gas. The example is :AgNO₃(aq) + NaCl(aq) ----> AgCl(s) + NaNO₃(aq)
HNO₃(aq) + KOH(aq) ----> KNO₃(aq) + H₂O(l)
To learn more about double displacement here
https://brainly.com/question/30048320
#SPJ4
which of the following elements is the most difficult to ionize? select the correct answer below: fr h he xe feedback more instruction submit content attribution- opens a dialog
Answers
The most difficult element to ionize among the options provided is helium (He).
Chemical element helium has the atomic number 2 and the symbol He. It is the first member of the noble gas group in the periodic table and is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas.[a] Its melting point at ordinary pressure is zero, and its boiling point is the lowest of all the elements. After hydrogen, it is the second-lightest and second-most prevalent element in the observable universe. More than 12 times the mass of all the heavier elements put together, it makes up around 24% of the total mass of the elements. Because of the extremely high nuclear binding energy (per nucleon), its abundance is comparable to that of the Sun and Jupiter.
To know more about noble gas:
https://brainly.com/question/29503115
#SPJ4
Which animals/insects look different from their young?
Answers
How are energy and work related? A. Energy is the force needed to do work B. Work times energy is force C. Energy is the capacity to do work D. Work and energy are the same
Answers
Answer:
I believe the answer is A
Explanation:
Work and energy are related because when you work, you cause displacement in the object you are exerting upon. While this happens, you transfer energy between the systems. Both work and energy share the same SI unit, called the joule.
Why are engineers called applied scientist?
Answers
Answer:
Applied science is a discipline that is used to apply existing scientific knowledge to develop more practical applications, for example: technology or inventions. In natural science, basic science (or pure science) is used to develop information to explain phenomena in the natural world.
Answer:
• Because engineers apply science and its theories into practical situation
Take an example of;
→ A generator: When creating a generator, electromagnetic induction theorem is applied. [ self and mutual inductance ]
→ A plane: The wings are made in considerance of Bernoulli principle and viscous drag
\(.\)
What is the best description of the results of a chemical reaction
Answers
Answer:
the final reaction
Explanation:
a chemical reaction is caused from the two reactants
The speed of a wave on a rope is 50cm/s an its wave
length is 10cm. What is its frequency
Answers
Answer:
Speed = frequency x wavelength 50 cm/s = Freq x 10cm
Explanation:
Which activity is the best demonstration of J.J. Thomson’s use of creativity in his work?
Answers
Answer:
The correct answer is making observations in his experiment. In his cathode ray tube experiment J. J. Thomson proved that the cathode rays are indeed negatively charged particles.
i will give Brainliest!!! What kind of atoms make up C12H22O11?
Answers
There are 12 Carbon atoms, 22 Hydrogen atoms and 11 Oxygen atoms.
Select the net ionic equation for the reaction of rubidium hydroxide with nitric acid. a. RbOH(aq) → Rb'(aq) + OH-(aq) b. HNO(aq) → H.(aq) + No,tag) c. Rb"(aq) + NO,-(aq) → RbNO(ag) d. H.(aq) + OH-(aq) → H2O(?) e. Rb-(aq) + OH-(aq) + H"(aq) + NOTaq)
Answers
The net ionic equation for the reaction of rubidium hydroxide with nitric acid is option e. Rb-(aq) + OH-(aq) + H"(aq) + NOTaq).
Like a molecular equation, which expresses compounds as molecules, an ionic equation is a chemical equation wherein the electrolytes in aqueous solution are expressed as dissociated ions.
An ionic equation is a chemical equation wherein the electrolytes in aqueous answer are written as dissociated ions. instance: 1) Sodium chloride(aq) + silver nitrate(aq) → silver chloride(s) + sodium nitrate(aq) >>Ag+(aq) + Cl-(aq) → AgCl(s) 2) Sodium(s) + hydrochloric acid(aq) -> sodium chloride(aq) + hydrogen(g).
Learn more about ionic equation here:-https://brainly.com/question/13879496
#SPJ4
A 1.0 L buffer solution is 0.250 M HC2H3O2 and 0.050M NAC2H3O2 .Which of the following actions will destroy the buffer effectiveness?
A. adding 0.050 moles of NaC2H3O2
B. adding 0.050 moles of NaOH
C. adding 0.050 moles of HCl
D. adding 0.050 moles of HC2H3O2
E. none of the above
Answers
The correct answer is B. Adding 0.050 moles of NaOH will destroy the buffer effectiveness in buffer solution.
A buffer solution is a solution that can resist changes in pH when small amounts of acid or base are added to it. In this case, the buffer solution consists of acetic acid (HC2H3O2) and its conjugate base (C2H3O2- or NAC2H3O2).
To determine if an action will destroy the buffer effectiveness, we need to consider what happens to the buffer components and how they affect the pH of the solution.
A. Adding 0.050 moles of NaC2H3O2: This will not destroy the buffer effectiveness because we are adding more of the conjugate base, which can react with any added acid to maintain the pH.
B. Adding 0.050 moles of NaOH: This will destroy the buffer effectiveness because the added base will react with the weak acid (HC2H3O2) to form water and the acetate ion (C2H3O2-), which is a strong conjugate base. This will shift the equilibrium towards the products and decrease the concentration of the weak acid, making it less effective at resisting changes in pH.
C. Adding 0.050 moles of HCl: This will not destroy the buffer effectiveness because we are adding acid, which can be neutralized by the buffer components.
D. Adding 0.050 moles of HC2H3O2: This will not destroy the buffer effectiveness because we are adding more of the weak acid, which can react with any added base to maintain the pH.
E. None of the above: This is not the correct answer because option B (adding 0.050 moles of NaOH) will destroy the buffer effectiveness.
Learn more about buffer solution here:
https://brainly.com/question/17164774
#SPJ11
What is the longest exothermic reaction?
Answers
Answer:intermetallic and thermite reactions.
Explanation:
.
A compound has a percent composition of 15.24% sodium (molar mass = 22.99
g/mol), 52.95% bromine (molar mass = 79.90 g/mol), and 31.81% oxygen (molar
mass = 16.00 g/mol). Assuming that the mass of the compound is 100 g, what is
the compound's empirical formula?
Show all your work. Please use correct formatting for subscripts and exponents. The math for-
Answers
The empirical formula of the compound obtained from the question given is NaBrO₃
Data obtained from the question Sodium (Na) = 15.24%Bromine (Br) = 52.95%Oxygen (O) = 31.81%Empirical formula =? How to determine the empirical formulaThe empirical formula of the compound can be obtained as illustrated below:
Divide by their molar mass
Na = 15.24 / 22.99 = 0.663
Br = 52.95 / 79.90 = 0.663
O = 31.81 / 16 = 1.988
Divide by the smallest
Na = 0.663 / 0.663 = 1
Br = 0.663 / 0.663 = 1
O = 1.988 / 0.663 = 3
Thus, the empirical formula of the compound is NaBrO₃
Learn more about empirical formula:
https://brainly.com/question/24297883
1.Compare Endothermic reactions to exothermic reactions (Define each and list 2 characteristics for each. Fill in the table below.-ENDOTHERMIC -EXOTHERMIC•Positive H Value •Negative H •ValueAbsorbs heat •Releases Heat•Products have higher energy •Products have a lower energy
Answers
When the enthalpy is positive (H > 0), the reaction is endothermic, that is, it absorbs energy.
When the enthalpy is negative (H< 0), the reaction is exothermic, that is, it releases energy.
Therefore, the correct matches are
Endothermic:
• Positive H Value.
,• Absorbs Heat.
,• Products have higher energy.
Exothermic:
• Negative H Value.
,• Releases Heat.
,• Products have lower energy.
The wall of the flask is periodically rinsed with the previously boiled, deionized water from the wash bottle. Does this titrimetric technique result in an increase, a decrease, or have no effect on the reported percent acetic acid in the vinegar
Answers
The titrimetric technique will have no effect on the reported percent acetic acid in vinegar despite periodically rinsing the wall of the flask with previously boiled, deionized water from the wash bottle.
Acetic acid is a common component in vinegar, which can be measured by titration. It is a quantitative chemical analysis method in which a solution of unknown concentration reacts with a standard solution of known concentration, typically until completion. It will cause the volume of the solution to be a slightly larger, and the concentration will be lowered when distilled or deionized water is added to the solution.
However, as long as the vinegar is measured against a known, unchanging standard, such as NaOH, the percent acetic acid reported in the vinegar would not change.
Rinsing the flask's walls with deionized water helps to ensure that the acetic acid reacts with the NaOH in the titration, and it reduces the risk of losing the NaOH and changing the final result. The volume of the vinegar sample used in the titration would be precisely measured, ensuring that the accurate amount of NaOH solution was used.
Learn more about Deionized water here,
https://brainly.com/question/6025332
#SPJ11
What is the volume of one nanocontainer?
Answers
The volume of one nanocontainer is the amount of space it can hold. To find the volume, you need to know the dimensions of the nanocontainer.
If the nanocontainer is a regular shape, such as a cube or a cylinder, you can use the appropriate formula to calculate the volume. For example, the volume of a cube is found by multiplying the length, width, and height of the cube. If the nanocontainer is an irregular shape, you can use water displacement to find the volume. Here's how it works:
1. Fill a graduated cylinder with a known volume of water.
2. Carefully place the nanocontainer into the cylinder, making sure it is fully submerged.
3. Measure the new volume of the water in the graduated cylinder.
4. The difference between the initial and final volume is the volume of the nanocontainer.
Remember to record your measurements accurately to get an accurate volume.
To know more about volume visit:
https://brainly.com/question/28058531
#SPJ11
What is the triangle law of vector quantity
Answers
Answer:
A law which states that if a body is acted upon by two vectors represented by two sides of a triangle taken in order, the resultant vector is represented by the third side of the triangle.
Explanation:
anyone know how to do this?
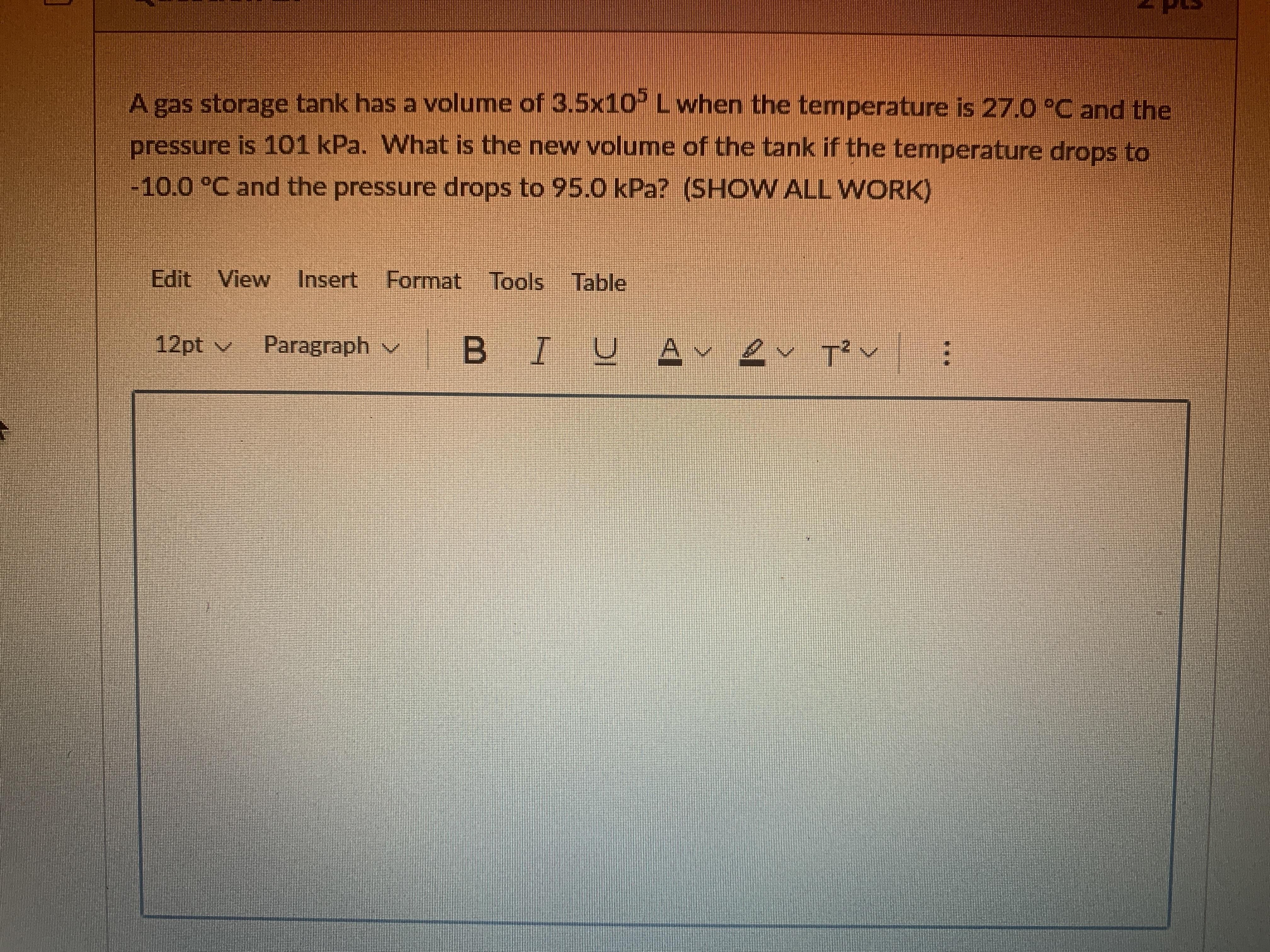
Answers
Answer:
3.26×10⁵ L
Explanation:
From the question given above, the following data were obtained:
Initial volume (V₁) = 3.5×10⁵ L
Initial temperature (T₁) = 27 °C
Initial pressure (P₁) = 101 KPa
Final temperature (T₂) = –10 °C
Final pressure (P₂) = 95 KPa
Final volume (V₂) =?
Next, we shall convert celsius temperature to Kelvin temperature. This can be obtained as follow:
T(K) = T(ºC) + 273
Initial temperature (T₁) = 27 °C
Initial temperature (T₁) = 27 °C + 273
Initial temperature (T₁) = 300 K
Final temperature (T₂) = –10 °C
Final temperature (T₂) = –10 °C + 273
Final temperature (T₂) = 263 K
Finally, we shall determine the new volume of the tank. This can be obtained as follow:
Initial volume (V₁) = 3.5×10⁵ L
Initial temperature (T₁) = 300 K
Initial pressure (P₁) = 101 KPa
Final temperature (T₂) = 263 K
Final pressure (P₂) = 95 KPa
Final volume (V₂) =?
P₁V₁ / T₁ = P₂V₂ / T₂
101 × 3.5×10⁵ / 300 = 95 × V₂ / 263
117833.333 = 95 × V₂ / 263
Cross multiply
95 × V₂ = 117833.333 × 263
95 × V₂ = 30990166.6
Divide both side by 95
V₂ = 30990166.6 / 95
V₂ = 3.26×10⁵ L
Thus, the new volume of the tank is 3.26×10⁵ L
Explain how to count the number of elements in a compound.
Answers
Answer:
Simply identify what elements are in a compound
Explanation:
For example in NaCl we have sodium (Na) and Chlorine (Cl)
In order to do this you would need to recognise the symbols for a certain element: O for oxygen; N for nitrogen; H for hydrogen etc.
What are water turbines used for?
Answers
Answer:
A water turbine is used to convert the energy contained in water, potential energy or kinetic energy, into mechanical or electrical energy. There are two types of water turbines, the reaction water turbine, and the impulse water turbine.
Explanation:
Answer:
Water turbines are used to take energy whether it may be kinetic or potential from the water and convert that energy into mechanical, or electrical energy.
Explanation:
Use the electron-transfer method to balance the equation involving the reaction of lithium with water to produce lithium hydroxide and hydrogen gas.
Answers
Answer:
Explanation:
The equation for the reaction of lithium with water to produce lithium hydroxide and hydrogen gas is:
Li + H2O -> LiOH + H2
To balance this equation using the electron-transfer method, we first need to determine the oxidation states of each element in the reactants and products.
In Li, the oxidation state of lithium is +1. In H2O, the oxidation state of hydrogen is +1 and oxygen is -2.
In LiOH, the oxidation state of lithium is +1, oxygen is -2 and hydrogen is +1.
In H2, the oxidation state of hydrogen is +1.
It's clear that lithium loses 1 electron to become Li+ and hydrogen gains 1 electron to become H-.
So, we have to add an electron on the right side of the equation to balance the electrons.
Li + H2O -> LiOH + H2 + e-
Now, the number of electrons on both sides of the equation is the same and the equation is balanced.
Alternatively, we can use the oxidation number method, where we balance the charge of the reactant and product side of the equation. The sum of the oxidation numbers of all atoms on the reactant side must equal the sum of the oxidation numbers of all atoms on the products side. In this case, the oxidation state of lithium is +1, hydrogen is +1 and oxygen is -2.
Li + H2O -> LiOH + H2
+1 +1 -2 = +1 +1 +1 -2
The equation is already balanced with respect to oxidation numbers
How does Benedict's solution react when a very high amount of reducing sugar is present in a sample? (Circle the correct answer.) a. Solution remains blue and no precipitate forms. b. Brick-red precipitate is formed. c. Yellow precipitate is formed. d. Yellowish-orange precipitate is formed.
Answers
When a very high amount of reducing sugar is present in a sample, Benedict's solution will react by forming a brick-red precipitate. The answer is B. Brick-red precipitate is formed.
The presence of this precipitate indicates a positive result for the test and indicates that the sample contains a significant amount of reducing sugar. This reaction is an example of an oxidation-reduction reaction in which the reducing sugar is oxidized and the Benedict's solution is reduced.
The reaction is as follows:
Reducing Sugar + Benedict's Solution → Brick-red Precipitate + Oxidized Benedict's Solution
Learn more about Benedict's solution at: https://brainly.com/question/11122902
#SPJ4
The graph below shows how solubility changes with temperature.
A graph with the horizontal axis showing temperature ranging from 0 to 10 in units of 10 and the vertical axis solubility in grams of salt per 100 grams of water. Several compounds are shown. All data are approximate. The substances and their coordinates are as follows: upper N a upper C l: 0, 38; 10, 38; 20, 38; 30, 38; 40, 39; 50, 39; 60, 39; 70, 40; 80, 40; 90, 40; 100, 40. Upper N a subscript 2 upper H upper A s upper O subscript 4: 0, 5; 10, 18; 20, 28; 30, 39; 40, 49; 60, 65; 80, 82. Upper B a (upper N upper O subscript 3) subscript 2: 0, 5; 10, 8; 30, 12; 40, 15; 50, 18; 60, 20; 80, 28; 100, 33. Upper N a subscript 2 upper S upper O subscript 4: 0, 5; 5, 8; 10, 10; 15, 15; 20, 20; 25, 20; 28, 35; 30, 40; 32, 49; 33, 50; 35, 50; 40, 48; 50, 47; 60, 46; 70, 45; 80, 43; 90, 32; 100, 40. Upper C 3 subscript 2 (upper S upper O subscript 4) subscript 3 dot 9 upper H subscript 2 upper O: 0, 18; 20, 10; 30, 8; 50, 5; 60, 3; 80, 1; 100, 0.
How many grams of NaCl will be needed to form 600 mL of a saturated solution at 100°C?
15 g
24 g
40 g
240 g
answer 240g
Answers
The amount of NaCl required to prepare a 600 mL saturated solution depends on the solubility at the temperature.
What is solubility?Solubility is the amount of solute that dissolves in a given volume of solvent.
Solubility = amount of solute/volume of solvent oSolutions may be saturated, unsaturated, super saturated solution.
For solutes, the solubility increases with increase in temperature.
The amount of NaCl required to prepare a 600 mL saturated solution depends on the solubility at the temperature.
Learn more about solubility at: https://brainly.com/question/23946616
Answer:
240
Explanation:
How many atoms are present in 7.50 mol of chlorine atoms? _
Answers
Answer:
4.52 x 10²⁴ atoms Cl
Explanation:
A mole is a name that means a certain number like a dozen means 12. 1 mole of chlorine atoms is 6.022 x 10²³ chlorine atoms. The unit conversion is 6.022 x 10²³/mol.
\(7.50molCl*\frac{6.022 x 10^{23} atomsCl}{1molCl} = 4.5165*10^{24} atomsCl\)
Round to 4.52 x 10²⁴ atoms Cl for the correct number significant figures.
A student dissolves 12.48 g of bluestone (CuSO4.5H₂O) in sufficient water to make up 200 mL of solution.
(a) What is the concentration of the solution?
(b) If the student takes 100 mL of this solution, what would be the concentration of the 100 mL
sample?
(c) If the 100 mL sample was then heated strongly to drive off all the water, what mass of copper(II)
sulfate residue would remain?
Answers
Since most reactions take place in solutions, it's critical to comprehend how the substance's concentration is expressed in a solution. The number of chemicals in a solution can be stated in a variety of ways including molarity.
The number of moles of dissolved solute per liter of solution is the definition of molarity, a unit of concentration. Molarity is defined as the number of millimoles per milliliter of the solution by dividing the number of moles and the volume by 1000.
The equation of molarity is:
M = Number of moles / Volume of solution in liters
a) n = 12.48 / 249.5 = 0.0500
M = 0.0500 / 0.2 = 0.25
b) M = 0.0500 / 0.1 = 0.5
c) Mass = n × Molar mass = 0.0500 × 249.5 = 12.47 g
To know more about molarity, visit;
https://brainly.com/question/16587536
#SPJ1
How many hydrogen atoms are in 2.0 GH
Answers
Answer:
2 hydrogen atoms
Explanation:
because the hydrogen atoms are two
what does it mean if the thermometer temperature suddenly drops in the middle of your distillation?
Answers
As the distillation head fills with the vapors of lower-boiling compound, the head temperature rises.
What happens to the temperature when you just distill something?
The temperature at which the solution must boil rises as the distillation goes on because the more volatile component boils out quicker. As a result, the distillate's composition varies with time. Because the lower-boiling compound completes the distillation process before the higher-boiling compound's vapors can fill the distillation head and raise the head temperature, the temperature lowers. The lower boiling liquid has finished its distillation process, leaving just the upper boiling liquid, which causes the temperature to begin to fall.
As a result, the impure liquid must be heated up in the flask it is contained in before it can begin to evaporate and reach the thermometer on top.
To learn more about simple distillation refers to:
brainly.com/question/24553469
#SPJ4