the complete combustion of 0.441 g of a snack bar in a calorimeter (ccal = 6.15 kj/°c) raises the temperature of the calorimeter by 1.63 °c. calculate the food value (in cal/g) for the snack bar.
Answers
The food value (in cal/g) for the snack bar can be calculated using the given information. The food value (in cal/g) for the snack bar is 1.623 cal/g.
Given that the mass of the snack bar, m = 0.441 g The calorimeter constant, ccal = 6.15 kj/°cThe rise in temperature of the calorimeter, ΔT = 1.63 °c We know that the heat evolved by the combustion of the snack bar is absorbed by the calorimeter. Hence, the heat evolved by the combustion of the snack bar = Heat absorbed by the calorimeter From the formula, Q = m × c × ΔTwhere,Q = Heat evolved by the combustion of the snack bar, and c = Specific heat capacity of water = 1 cal/g °c Now,Q = m × c × ΔT = 0.441 g × 1 cal/g °c × 1.63 °c= 0.717cal
Thus, the heat evolved by the combustion of the snack bar is 0.717 cal. Now, the food value of the snack bar (in cal/g) can be calculated by dividing the heat evolved by the mass of the snack bar. Food value = Heat evolved / mass of snack bar= 0.717 cal / 0.441 g= 1.623 cal/g Therefore, the food value (in cal/g) for the snack bar is 1.623 cal/g.
To know more about calorimeter refer to:
https://brainly.com/question/30873343
#SPJ11
Related Questions
How are a carrot, an amoeba, and a bacterium alike?
They are all made of cells.
They are eukaryotes.
They are prokaryotes.
They all live in soil.
Answers
Answer:
They all are made up of cells
Answer: They are all made of cells.
Explanation:
PLZ HELP!!! QUESTIONS ARE BELOW!!! :D PLZ COMPLETELY ANSWER THE QUESTIONS WITH A GOOD ANSWER :)

Answers
Answer: sorry its lagging i hope this help fore 18.. =D
Explanation:
n,s is repeating the cycle of the inside
Answer/Explanation:
18, a,
I believe the n, and s represents North, South, or just the way the arrows are rotating repeatedly.
18, b,
The phenomenon re-occurring shows the alignment of magnetic dipoles with in a magnet, like inside. The arrows show a direction in the line with an external magnetic field in a way that it can produce its own magnetic fields that seem to travel from North to the South.
18, c,
Incorrect. Refrigerator magnets have multiple layers of magnification, they use opposite polarities to stay this way. If he puts the magnet sideways it may stick to the fridge
~ LadyBrain
which of the following is the strongest acid? ch3c||ooh ch3ch2oh ch3c||oh ch3och3 ch3c||ooch3
Answers
Among the given compounds, the strongest acid is CH3COOH. It is also known as acetic acid or ethanoic acid.
Acetic acid is a weak acid, but it is stronger compared to the other compounds listed. CH3COOH has a carboxylic acid functional group (-COOH) which can donate a hydrogen ion (H+) in solution, making it an acid. This functional group contains an electronegative oxygen atom that can stabilize the negative charge on the conjugate base, resulting in a more acidic compound.
On the other hand, the other compounds listed do not possess an acidic functional group. CH3CH2OH is ethanol, which is a neutral molecule. CH3C||OH is methyl alcohol or methanol, which is also a neutral molecule. CH3OCH3 is dimethyl ether, a neutral compound. CH3C||OOCH3 is methyl acetate, which is a neutral ester. Therefore, among the options provided, CH3COOH (acetic acid) is the strongest acid.
Learn more about Acetic acid here:
https://brainly.com/question/4300470
#SPJ11
Which of these equations are balanced
Answers
Answer:
Post the equations for help :)
Explanation:
Ethylenediamine (en) is a bidentate ligand. What is the coordination number of cobalt in [Co(en)2Cl2]Cl
Answers
The coordination number of cobalt in [Co(en)₂Cl₂]Cl is 6.
The formula [Co(en)₂Cl₂]Cl indicates that there are two ethylenediamine (en) ligands, each of which can donate two electrons to the cobalt ion (Co), making a total of four electrons donated by the ligands.
Additionally, there are two chloride (Cl⁻) ions, each of which can donate one electron to the cobalt ion. Therefore, there are a total of six donor atoms surrounding the cobalt ion, which gives a coordination number of 6.
The coordination number of a metal ion is the number of donor atoms that are directly bonded to the metal ion. In this case, the ethylenediamine ligands are bidentate, meaning that they can form two bonds with the metal ion, and each chloride ion can form one bond with the metal ion.
Therefore, the total number of donor atoms surrounding the cobalt ion is six, which gives a coordination number of 6.
To know more about coordination number, refer here:
https://brainly.com/question/31610101#
#SPJ11
Draw the neutral organic product that results from the given reaction. Include all hydrogen atoms. CH_3CH_2CH_2OH(I) H_2SO_4 (conc) /200^∘C
Answers
The neutral organic product obtained from the reaction is propene (CH3CH=CH2)
What is the role of concentrated sulfuric acid in the dehydration of 1-propanol?The given reaction involves the dehydration of 1-propanol (CH3CH2CH2OH) in the presence of concentrated sulfuric acid (H2SO4) at 200°C. Dehydration is the removal of a water molecule from the alcohol compound. In this case, 1-propanol loses a water molecule to form propene (CH3CH=CH2), which is an alkene.
The neutral organic product resulting from this reaction is propene (CH3CH=CH2). The reaction occurs as the hydroxyl group (-OH) of 1-propanol is protonated by the acidic H2SO4, leading to the loss of a water molecule. This creates a double bond (C=C) between the second and third carbon atoms, resulting in the formation of propene.
Thus, the neutral organic product obtained from the reaction is propene (CH3CH=CH2).
Learn more about neutral organic product brainly.com/question/17313223
#SPJ11
A saline solution contains 0.90g of sodium chloride dissolved in 250 cm3 water
(a)what is the amount in moles of sodium chloride dissolved?
(b) the concentration of the saline solution in mol/dm3?
Answers
Explanation:
A. mole=mass/molar mass
Molar mass of NaCl=23+35.5g
NaCl=58.5g
from
mole=0.90/58.5mol
mole=0.90g/58.5mol
mole=0.15
B. concentration=number of moles of solute/volume of solution
concentration=0.15mol/250cm^3
1dm^3=1000cm^3
250=0.25
concentration=0.6mol/dm^3
Calculate the energy required to heat 712.0 g of graphite from 2.0 °C to 20.7 °C. Assume the specific heat capacity of graphite under these conditions is 0.710 J-g-K Round your answer to 3 significant digits.
Answers
To calculate the energy required to heat a substance, we can use the formula:
Q = mcΔT
where Q is the heat energy, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
Given that we have 712.0 g of graphite, a specific heat capacity of 0.710 J/g-K, and a temperature change of 18.7 °C (20.7 °C - 2.0 °C)
Q = (712.0 g) x (0.710 J/g-K) x (18.7 °C)
Q = 10,965.64 J.
The energy required to heat 712.0 g of graphite from 2.0 °C to 20.7 °C is 10.965 kJ.
Learn more about Specific Heat Capacity here: https://brainly.com/question/29766819
#SPJ4
Magnetic fields all have the same or equal strength .
true or false ?
Answers
Use the Word Correctly in a Sentence
Ion
Answers
by adding sds (sodium dodecyl sulfate) during the electrophoresis of proteins, it is possible to:
Answers
By adding sodium dodecyl sulfate (SDS) during the electrophoresis of proteins, it is possible to denature the proteins and give them a negative charge, making them separate based on size during electrophoresis.
SDS is an anionic detergent that binds to proteins and unfolds them, resulting in a uniform negative charge distribution along the length of the protein. This allows the proteins to migrate through the gel matrix based solely on their molecular weight, rather than their shape or net charge. The SDS-PAGE technique, which uses SDS as a key reagent, is widely used for the separation and analysis of proteins. During SDS-PAGE, the protein samples are first denatured and treated with SDS, then loaded into wells of a polyacrylamide gel, and subjected to an electric field. As a result, the proteins migrate through the gel in proportion to their molecular weight, with smaller proteins moving faster and larger proteins moving slower. The separated proteins can then be visualized and analyzed using various staining and detection methods.
Learn more about electrophoresis here:
https://brainly.com/question/28014593
#SPJ11
How many moles are there in 17.6 grams of NaOH, sodium hydroxide?
Answers
Answer: moles= 0.44moles
Explanation:
Moles = mass/molar mass
Molar mass of NaOH= 23+16+1= 40gmol-1
n= 17.6/40= 0.44 moles
i hope i helped u
Water near the surface of a tropical ocean has a temperature of 298.2 K(250
∘
C), whereas water 700 m beneath the surface has a temperature of 280.2 K(7.0
∘
C). It has been proposed that the warm water be used as the hot reservoir and the cool water as the cold reservoir of a heat engine. Find the maximum possible efficiency for such an engine. Analytical solution is give. Convert the same into the necessary generalized function [4]
Answers
The maximum possible efficiency for such an engine is 6.039 %.
Temperature of water near the surface of a tropical ocean = 298.2 K
Temperature of water 700 m beneath the surface = 280.2 K
To find the maximum possible efficiency for the given heat engine,
The maximum possible efficiency of a heat engine depends only on the temperatures of the hot and cold reservoirs, and is given by Carnot efficiency, η = (T₁ - T₂)/T₁
whereT₁ is the temperature of hot reservoir, T₂ is the temperature of cold reservoir. Temperature is given in Kelvin.
The temperature difference between the hot and cold reservoirs is, T₁ - T₂ = 298.2 K - 280.2 K = 18 K
Substitute these values in the Carnot efficiency equation,
Carnot efficiency, η = (T₁ - T₂)/T₁ = (18 K)/298.2 K = 0.06039. The maximum possible efficiency for such an engine is 6.039 %.
Generalised function is given as η = (T₁ - T₂)/T₁
Learn more about efficiency of Carnot engine: https://brainly.com/question/27359482
#SPJ11
WHAT HAPPENS WHEN ELECTRIC CURRENT PASSES THROUGH ACIDIFIED WATER.
Answers
Answer:
When an electric current is passed through acidified water, it decomposes to give hydrogen and oxygen gas. The hydrogen gas is obtained at the cathode and the oxygen gas is obtained at the anode
identify the δh and δs for the following physical change of br2. br2(g) → br2(g)
Answers
The enthalpy change (ΔH) for the physical change of Br2 from the gas phase to the gas phase (Br2(g) → Br2(g)) is zero. The entropy change (ΔS) for this physical change is also zero.
In a physical change, the chemical substance remains the same, and there is no breaking or forming of chemical bonds. In the case of Br2 going from the gas phase to the gas phase, there is no change in the chemical identity or composition of the substance.
The enthalpy change (ΔH) measures the heat energy transfer during a reaction or process. Since there is no change in the chemical bonds or composition of Br2 in this physical change, there is no transfer of heat energy, and thus ΔH is zero.
The entropy change (ΔS) quantifies the degree of disorder or randomness in a system. In this physical change, the arrangement and distribution of Br2 molecules remain unchanged, leading to no change in entropy. Therefore, ΔS is also zero.
To learn more about enthalpy change (ΔH) visit: brainly.com/question/17285535
#SPJ11
a 22.4 l container at 0 oc contains 0.30 mol n2, 0.20 mol o2, 0.40 mol he and 0.10 mol co2.what is the partial pressure (in atm) of oxygen?
Answers
The partial pressure of oxygen is 0.49 atm.
To find the partial pressure of oxygen, we need to first calculate the total pressure of the mixture using the ideal gas law:
PV = nRT
where P is pressure, V is volume, n is the number of moles, R is the gas constant, and T is temperature in Kelvin.
Rearranging this equation, we get:
P = nRT/V
Substituting the given values, we have:
P = (0.30 + 0.20 + 0.40 + 0.10) mol x 0.0821 L·atm/mol·K x 273 K / 22.4 L
= 2.44 atm
The partial pressure of oxygen can be calculated by multiplying the mole fraction of oxygen by the total pressure:
moles of O2 = 0.20 mol
moles of all gases = 0.30 + 0.20 + 0.40 + 0.10 = 1.00 mol
mole fraction of O2 = moles of O2 / moles of all gases = 0.20 / 1.00 = 0.20
partial pressure of O2 = mole fraction of O2 x total pressure = 0.20 x 2.44 atm = 0.49 atm
Therefore, the partial pressure of oxygen is 0.49 atm.
To know more about partial pressure, refer here:
https://brainly.com/question/31214700#
#SPJ11
The teacher was not in the room yet. Randy began opening containers of chemicals, touching them with his hands. His cheek itches so he rubbed it. Explain how Randy was not acting safety in the the laboratory.
I know the answer I just don’t know how to word it. Please help. Due by 11:59 tonight
Answers
Answer:
Randy went into the classroom without the teacher being present. He opened containers of chemicals without the teacher's consent. Randy is making contact with the chemicals on his skin.
I hope this helps! I haven't done lab science in a while so I'm trying to remember the safety precautions.
Randy went into the classroom without the teacher being present and opened containers of chemical, making contact with chemicals on skin leads to skin reaction.
So Randy should follow the lab safety rules while entering into lab.
What are the important lab safety rules?After entering lab wear apron and gloves along with safety goggles if working with any hazardous or infectious substance.
Fully aware about the hazards of the materials, properly trained to handle lab equipment, chemicals should be adequately labelled.
Taste or smell chemicals are prohibited, never pour already stored chemicals, chemical hygiene plan should be developed for specific hazards.
Avoid to adopt un-tested and unauthorized experimentation, Clean up all chemical spill, Laboratory induction should be carried out .
Avoid storage of any edible items in the refrigerator along with lab chemicals and specimens, clean the work station after completing experiment and sterilization should be done before starting experiment.
Learn more about lab safety rules , here:
https://brainly.com/question/5147285
#SPJ2
write the structure of 2 butene class 10
Answers
Answer:
The answer is C4H8.
Explanation:
I hope it helps you
Pass your test fast okay
BYE!
I WILL GIVE U BRAINLIEST IF UR CORRECT
Which of the following is always a result of a chemical reaction?
Hint: The questions says always.. only one best answer
A. Bubbles
B. Fire or smoke
C. Temperature change
D. A new substance
Answers
Answer:
D: a new substance
Explanation:
Chemical reaction
A change in matter that produces on or more new substances
Answer:
D. A new substance is always a result of a chemical reaction.
Explanation:
In a chemical reaction, one or more reactants reacts/decomposes to form one or more products.
Choose all of the following that are FALSE.
A. Paper makes up the largest proportion of MW in the United States.
B. If you wash your plastic bottles with warm water that was heated via coal-generated electricity before recycling them, then recycling your plastic bottles could release more carbon into the atmosphere than throwing them
away.
C. Total waste generation in the United States has been steadily increasing since about 1950. Globally. D. solid waste management costs are expected to begin decreasing as waste management
technology gets cheaper.
Answers
A. Paper makes up the largest proportion of MW in the United States. (False) C. Total waste generation in the United States has been steadily increasing since about 1950. Globally. (False)
D. Solid waste management costs are expected to begin decreasing as waste management technology gets cheaper. (False)
The false statements are A, C, and D.
A. Paper does not make up the largest proportion of municipal waste (MW) in the United States. While paper waste is significant, it is not the largest component. Other materials like food waste, plastics, and metals also contribute to MW.
C. Total waste generation in the United States has not been steadily increasing since about 1950. In fact, waste generation rates have fluctuated over the years due to various factors such as population growth, consumption patterns, and waste management practices.
D. Solid waste management costs are not expected to decrease as waste management technology gets cheaper. While advancements in technology can lead to more efficient waste management processes, they often come with their own costs, such as implementation, maintenance, and regulatory compliance. These factors can offset any potential cost savings and may even lead to an increase in waste management costs over time.
learn more about Solid waste management here:
https://brainly.com/question/14665452
#SPJ11
why are fish lucky that water particles expand as they hit a temperature of 0°c?
Answers
Answer:
it is a result of hydrogen bonds present within water molecules.
Explanation:
when the water is transformed to ice at zero degrees Celsius, the water molecules are in crystal lattice in a structure that has a lot of empty space around each molecule.
Why is it important to pasteurize certain liquids?
Answers
Answer:
Pasteurization involves heating liquids at high temperatures for short amounts of time. Pasteurization kills harmful microbes in milk without affecting the taste or nutritional value (sterilization= all bacteria are destroyed).
Explanation:
what ocurrrs when the vapor pressure of a liquid is equal to the external atmospheric pressure
Answers
Answer:
The change from a liquid phase to a gaseous phase.
so the answer would be it changed to a gaseous phase
---------------
This is what occurs when the vapor pressure of the liquid is equal to the atmospheric pressure exerted on the liquid.
1) What is the mass in grams of a 51.2-mole sample of calcium?
Answers
Answer:
2051.9936 g
Explanation:
Mass = ?
Number of moles = 51.2 mol
The relationship between both quantities is given by the equation;
Number of moles = Mass / Molar mass
Solving for mass;
Mass = Number of moles * Molar mass
Mass = 51.2 * 40.078
Mass = 2051.9936 g
This graph represents an endothermic reaction. What does it show about the potential energy of reactants and products? Abuzua nunuad in Reaction progress A. The comparison of potential energy depends on what the reactants and products are, B. The potential energy of the products is greater than the potential energy of the reactants C. The potential energy of the reactants equals the potential energy of the products D. The potential energy of the reactants is greater than the potential energy of the products,

Answers
Answer:
The potential energy of the products is greater than the potential energy of the reactants
Explanation:
i just took the test
In an endothermic reaction, activation potential energy of reactants is less than that of the products. Reactants in endothermic reaction are absorbing enough heat energy to overcome this barrier potential. Hence, option B is correct.
What is an endothermic reaction?An endothermic reaction is the one in which heat energy is absorbed by the reactants from the surroundings. In endothermic reactions, the enthalpy change is positive.
The minimum energy that the reactants have to acquire for effective collision and reaction is called the activation energy. By absorbing heat energy reactants becomes more energetic and overcome this activation potential.
Therefore, in an endothermic reaction, the activation potential of products will be higher than that of the reactants and the energy diagram clearly exhibit this transition in energy. Hence, option B is correct.
Find more on endothermic reaction:
https://brainly.com/question/23184814
#SPJ2
stated that acids taste ___________________, are corrosive to _____________________, change the color of litmus to _______________, and become less acidic when mixed with _______________.
Answers
Acids taste sour, are corrosive to metals and tissues, change the color of litmus to red, and become less acidic when mixed with bases.
Acids have a distinct taste of sourness. This taste is due to the presence of hydrogen ions (H+) in the acid that stimulate the sour receptors on our tongue. However, it's important to note that tasting acids can be dangerous and should never be done without proper safety measures.
Acids have a corrosive nature, meaning they can erode or damage certain materials like metals and tissues. This property is due to the ability of acids to donate hydrogen ions, which can react with the material's surface and break it down.
Litmus is a pH indicator that changes color in response to acidic or basic solutions. When an acid is added to litmus, it turns red, indicating the presence of an acidic solution. This color change occurs due to the acidic solution's ability to donate hydrogen ions that react with the litmus indicator.
When an acid is mixed with a base, it results in a neutralization reaction. In this reaction, the acid and base react to form water and a salt, ultimately reducing the acidity of the solution. This happens because the base accepts hydrogen ions from the acid, reducing the concentration of H+ ions and increasing the concentration of OH- ions.
To know more about acids click on below
link :
https://brainly.com/question/13586622#
#SPJ11
How are the vapor pressure and boiling point of alkynes affected as the chain length increases?
Answers
Answer:
Answer to the following question is as follows;
Explanation:
Alkynes' vapour pressure and normal boiling points are altered when chain length grows, since vapour pressure rises while boiling point falls.
Vapour pressure always include pressure entered by vapour with its condensed phase, pressure include molecules force of attraction include vapour
A LOT OF POINTS!!
30.0g sample of calcium sulfide contains 16.4g of calcium. What is the % of sulfur by mass in the compound
Answers
Answer:
there you go man
Explanation:


The relationship between the change in Gibbs free energy and the emf of an electrochemical cell is given by ________.
Answers
The relationship between the change in Gibbs free energy (ΔG) and the emf of an electrochemical cell is given by the equation ΔG = -nFE, where n is the number of moles of electrons transferred, F is the Faraday constant (96,485 C/mol), and E is the emf of the cell.
The relationship between the change in Gibbs free energy and the emf of an electrochemical cell is given by the equation ΔG = -nFE, where ΔG is the change in Gibbs free energy, n is the number of moles of electrons transferred, F is Faraday's constant (96,485 coulombs per mole of electrons), and E is the emf of the cell.
Visit here to learn more about Electrochemical:
brainly.com/question/10470515
#SPJ11
Does anyone know how to do this?
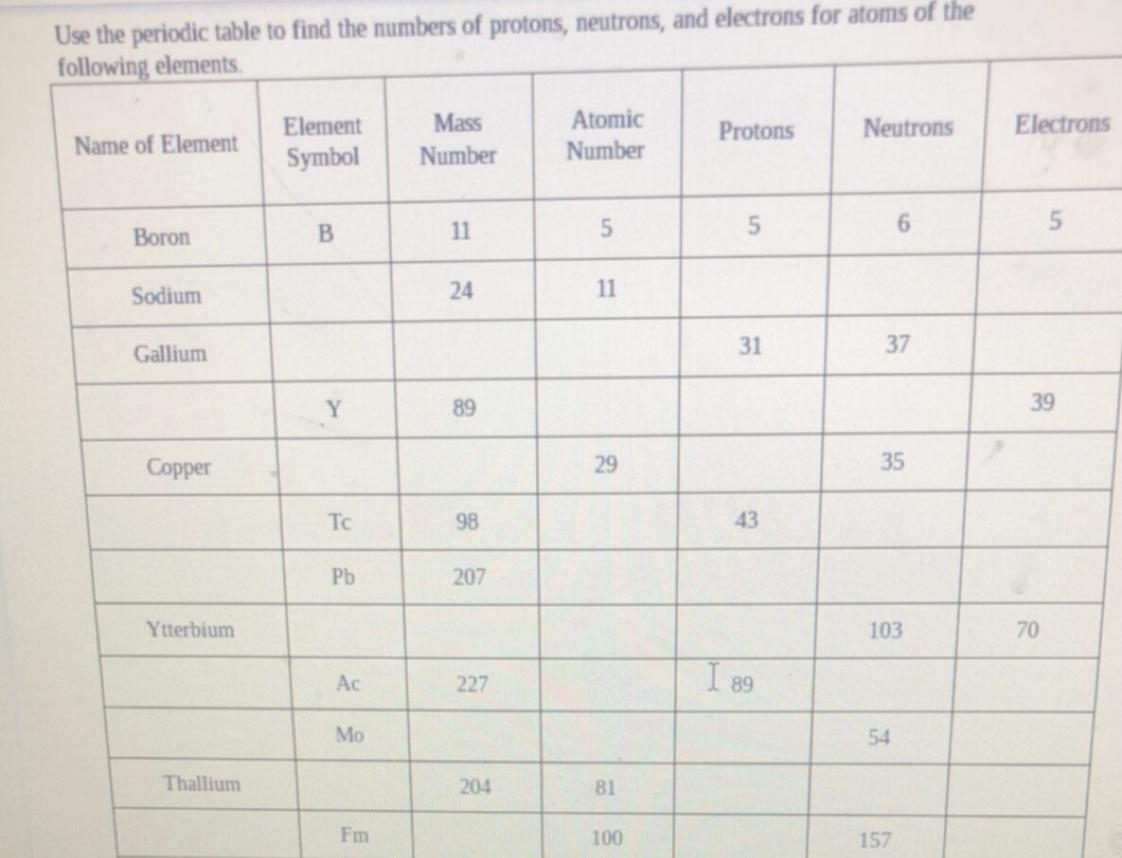
Answers
Answer:
yes
Explanation:
i don't have the periodic table in front of me right now but you will need it. for the first coloumn, look at the elemnt symbol and write it down. for example hydrogen H. the atomic mass will be written on the bottom of the entry. the atomic number is on the top. the number of protons is the atomic number. the neutrons is atomic mass-atomic number and the electrons you have to count the numbers in the upper right corner. depends what element you look at, there might be a couple rows of the electrons which are the shells. hope i helped :)