. The black, gooey liquid, which contains a mixture of combustible hydrocarbons along with small amounts of sulfur, oxygen, and nitrogen impurities and enters the refining process, is called ____ oil.
a. diesel
b. crude
c. heavy
d. proven
e. natural
Answers
The black, gooey liquid, which contains a mixture of combustible hydrocarbons along with small amounts of sulfur, oxygen, and nitrogen impurities and enters the refining process, is called crude oil (b)
Crude oil is a naturally occurring, unrefined petroleum product that is extracted from the ground. It is a mixture of various hydrocarbons, which are organic compounds made up of hydrogen and carbon. Crude oil is typically black or dark brown in color and has a thick, viscous texture.
Crude oil also contains small amounts of sulfur, oxygen, and nitrogen impurities, which are removed during the refining process. These impurities can have harmful effects on the environment and human health if they are not properly managed. Therefore, refining is an important process that transforms crude oil into useful products, such as gasoline, diesel fuel, and heating oil, while also reducing its environmental impact.
To know more about crude oil, visit:
https://brainly.com/question/4433699#
#SPJ11
Related Questions
Calculate the molarity of the two solutions. The first solution contains 0.650 mol of NaOH in 1.35 L of solution.
Answers
Answer:
0.481 M
Explanation:
The molarity ratio looks like this:
Molarity = moles / volume (L)
Since you have been given both the mass and volume, you can plug them into the equation to find the molarity.
Molarity = moles / volume
Molarity = 0.650 moles / 1.35 L
Molarity = 0.481 M
for number less than 0.1, such as 0.06, the zeros to the right of the decimal point but before the first nonzero digit
Answers
For the number less than 0.1 such as 0.006, the zeroes to the right of the decimal point but before the first non zero digit show the decimal place of the first significant digit.
The number that is given as digits is established using significant figures.Any two non-zero digits that are separated by a zero are significant figure.Every zero that is both to the right and left of a non-zero digit and the decimal point is not significant figure. The quantity of significant figures frequently reveals the degree of measurement accuracy. From the first non-zero digits in the figure, we may determine the number of significant figures.There is only one significant figure in the provided number 0.06. The decimal place of the first digit is indicated by the zeros that appear to the right of the decimal point but before the first non-zero digit.
Learn more about significant figure here:
https://brainly.com/question/11151926
#SPJ9
Vertical columns on the periodic table are known as
and horizontal rows are called
O periods, groups
groups, periods
O metals, nonmetals
O metalloids, metals
Answers
Answer:
groups and periods
Explanation:
which formulas represent ionic compounds and which represent covalent compounds?
a. NaCl, H2O
b. CH4, MgO
c. CO2, KBr
d. NH3, CaF2
Answers
The formula that represents ionic compounds is NaCl, MgO, KBr, and CaF2; while those that represent covalent compounds are H2O, CH4, CO2, and NH3. Option D is correct .
Ionic compounds are formed by the transfer of electrons, while covalent compounds are formed by sharing of electrons. An ion is an atom that has either gained or lost electrons to get a stable electron configuration. A positive ion is formed when an atom loses one or more electrons. A negative ion is formed when an atom gains one or more electrons. The structure of an ionic compound is typically crystalline.
A crystal lattice structure is made up of repeating patterns of ions. They have high melting and boiling points, do not dissolve in nonpolar solvents, and can be electrically conductive when dissolved in water. They are usually solids at room temperature.Covalent compounds are made up of non-metals. The atoms share electrons to create covalent bonds in a covalent compound. Each molecule in a covalent compound has a definite shape.
They have lower melting and boiling points than ionic compounds, dissolve easily in nonpolar solvents, and do not conduct electricity. They can exist as a gas, liquid, or solid at room temperature.
Learn more about ionic compounds
brainly.com/question/30420333
#SPJ11
Please help thank you

Answers
Answer:
The answer is the 2nd one down: The green house affect
a lizard basking in the morning sun is an example of
Answers
A lizard basking in the morning sun is an example of behavior known as thermoregulation. Thermoregulation is a behavior that allows animals to control their body temperature by adjusting to the surrounding environment.
A lizard basking in the morning sun is an example of this behavior, as it is a common way for reptiles to regulate their temperature. By absorbing heat from the sun, the lizard can increase its body temperature to an optimal level that is necessary for its metabolic processes to function properly. This helps the lizard maintain its energy levels and overall health, and is crucial for survival in a changing and unpredictable environment. Thermoregulation is an important adaptation that has evolved in many species to help them cope with variations in temperature, and is essential for their survival.
Learn more about Thermoregulation here: brainly.com/question/15276860
#SPJ4
which is the correct formula for the compound formed between beryllium and nitrogen?
Answers
Answer:
Be3N2
Explanation:
u cross multiply with their subscripts
A olvent i found to be 50. 0% oxygen, 37. 5% carbon, and 12. 5% hydrogen. What i the empirical formula of thi olvent
Answers
The empirical formula of the solvent is CH4.
Relative number of atoms
Of H= 25/1 = 25
Of C= 75/12 = 6.25
What is a solvent?
Solvents are a heterogeneous group of structurally different chemicals that can be used to dilute, dissolve, or disperse other compounds. The ability of a solvent to dissolve another molecule depends on the molecular structure and physical properties of both the solvent and the solute. Solvents can be categorized as organic or inorganic and in terms of chemical polarity. Polar solvents include water, alcohols, and other chemicals containing –OH, such as acetic acid, which have the ability to donate H+ and form hydrogen bonds. Polar solvents lacking the –OH group, including acetonitrile, dimethylformamide, and dimethylsulfoxide, are protophilic solvents and are used to dissolve less polar solutes.To know more about solvents and solutes, click the link given below:
https://brainly.com/question/17061863
#SPJ4
someone help me please....
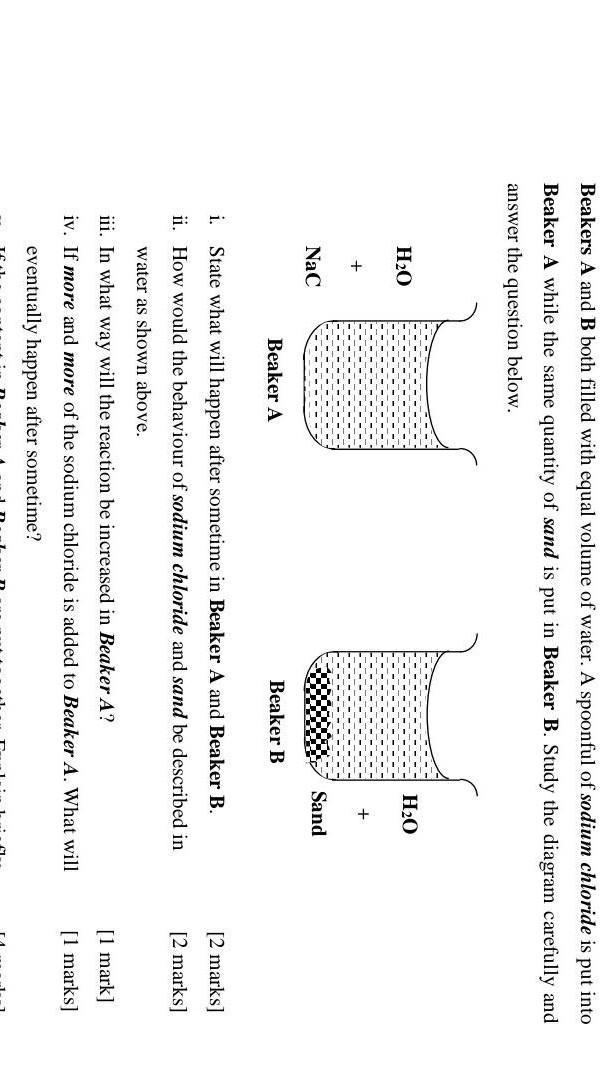
Answers
Answer:
i- In beaker A, sodium chloride will dissolve with water.
and in beaker B, there will be no reaction.
ii- sodium chloride is soluble in water, while sand is insoluble.
iii- The reaction can be increased by adding more sodium chloride to the beaker.
iv- The sodium chloride will no longer dissolve in the water.
How do you find the molar mass of ethanol?
Answers
The molar mass of ethanol is 46.069 g/mol because it has 2 mol of carbon atoms (2 12.011 g), 6 mol of hydrogen atoms (6 1.0079 g), and 1 mol of oxygen atoms (1 15.9994 g) in 1 mol.
Ethanol has a molar mass of 46 gmol-1.
Because you already know, molar mass refers to the entire mass of one molecule of a certain substance, and the basic chemical formula of ethanol is C2H5OH. So, if there are numerous atoms in a compound, we may calculate its molar mass by adding up the masses of its constituent atoms and multiplying the result by the number of atoms. We may calculate the molar mass of ethanol as 2x(molar mass of carbon) + 6x(molar mass of hydrogen) + 1x(molar mass of oxygen), or 2x12 + 6x1 + 1x16 = 24 + 16 + 6 = 46, since ethanol contains two carbon atoms, six hydrogen atoms, and one oxygen atom.
Learn more about Molar mass here:
https://brainly.com/question/22997914
#SPJ4
is water wet yes or no
Answers
Answer:
no water isn't wet.
Explanation:
Water isn't wet by itself, but it makes other materials wet when it sticks to the surface of them
Usually the HNMR is not used to analyze the % composition of mixtures. However, we used it for the cis and trans products. Explain what property of the product allows us to do that. (reduction lab)
Answers
The property of the product that allows us to use HNMR to analyze cis and trans products is the fact that the two products have different numbers of peaks in their spectra.
What is spectra ?Spectra is the range of all electromagnetic radiation, from the longest wavelengths (such as radio waves) to the shortest (such as gamma rays). It is a way of visualizing the amount of energy that is emitted at different frequencies and wavelengths. Spectra can be used to analyze light and other forms of electromagnetic radiation, such as X-rays and ultraviolet radiation. Spectra can also be used to study the composition and structure of stars, galaxies, and other astronomical objects. Spectra can also be used to identify elements and compounds, which can be used to study the makeup of a material or to detect the presence of certain substances.
To learn more about spectra
https://brainly.com/question/2416388
#SPJ4
helium is a gas at the boiling point of liquid nitrogen, 77.0 k. if 2.00 g of he is placed inside a 1.50 l container immersed in liquid nitrogen at 77.0 k, what is the pressure exerted by the helium gas?
Answers
The ideal pressure exerted by the helium gas is 426580 Pa.
We need to know about the ideal gas theory to solve this problem. The ideal gas is assumed that there is no interaction between particles in a gas. It can be determined by the equation
P . V = n . R . T
where P is pressure, V is volume, n is the number of moles gas, R is the ideal gas constant (8.31 J/mol.K) and T is temperature.
From the question above, we know that the parameters of Helium gas are
T = 77 K
m = 2 g
V = 1.5 L = 1.5 x 10¯³ m³
Mr He = 2
By substituting the given parameters, we can calculate the pressure
P . V = n . R . T
P . 1.5 x 10¯³ = 2 / 2 . 8.31 . 77
P = 426580 Pa
Find more on ideal pressure at: https://brainly.com/question/25290815
#SPJ4
1 Atoms of the same element with different numbers of neutrons are defined as:
o
neutrons
O
isotopes
O
elements
O
compounds
Answers
Answer:
The answer is B Isotopes
what is the ph of a 1.0 x 10–2-molar solution of hcn? (for hcn, ka = 4.0 x 10–10.) (a) 10 (b) between 7 and 10 (c) 7 (d) between 4 and 7 (e) 4
Answers
The pH of 1 X 10-2 M HCN acid is 5.7
D) between 4 and 7
HCN is weak acid dissociate as
HCN + H2O \rightleftharpoons H3O+ + CN-
Ka = [CN- ][H3O+] / [HCN]
but [CN- ] = [H3O+] = x
Ka = [x][x] / [HCN]
Substitute the value in equation
4.0X 10-10 = [x]2/ 1 X 10-2
[x]2 = 4.0X 10-10 X1 X 10-2 = 4.0 X 10-12
[x] = 2.0 X 10-6 M
Concentration of H3O+ = 2 X 10-6 M
pH = - log[H3O+]
pH = - log (2 X 10-6)
pH = 5.7
What is HCN?Prussic acid, also known as hydrogen cyanide, is a chemical substance with the formula HCN and the structural formula HCN. It is a colourless, incredibly poisonous, and flammable liquid that boils at 25.6 °C (78.1 °F), just slightly above room temperature.
Industrial-scale HCN production makes it a highly prized precursor to a wide range of chemical compounds, from pharmaceuticals to polymers. Production of potassium cyanide and adiponitrile, which are used in mining and plastics, respectively, has large-scale applications. Due to its volatile nature, it is more toxic than cyanide compounds that are solid.
Learn more about Hydrogen cyanide
https://brainly.com/question/27179806
#SPJ1
Determine the shape, ideal bond angle(s), and the direction of any deviation from those angles for each of the following:
(b) IF₄⁻
Answers
The shape is tetrahedral, ideal bond angle(s) is 60 degree, and the direction of any deviation is away from axis from those angles for IF₄⁻.
What is ideal bond angle?
Bond angles affect how a molecule is structured as well. Bond angles are the angles that connect adjacent lines to form bonds. The bond angle can be used to differentiate between linear, trigonal planar, tetrahedral, trigonal-bipyramidal, and octahedral structures. The VSEPR theory is supported by the ideal bond angles, which demonstrate the maximum angle at which repulsion would be minimized.
In essence, bond angles show that electrons prefer to be far apart. Electrons make up negative ions. The opposites don't attract one another. Now draw a contrast. You don't want to interact with a negative individual because they are often thought of as unpleasant or harsh.
To learn more about bond angle visit:
https://brainly.com/question/13751116
#SPJ4
21 The diagram shows an experiment. A B blue litmus paper D aqueous ammonium chloride What happens to the pieces of litmus paper? + aqueous sodium hydroxide blue litmus paper changes colour changes colour no colour change no colour change SHIRED heat red litmus paper changes colour no colour change changes colour no colour change red litmus paper
Answers
Three is no change in the blue litmus but the red litmus change color. This is because the ammonia turns red litmus blue due to ammonia.
What is the reaction of ammonium chloride and sodium hydroxide?When ammonium chloride and sodium hydroxide are mixed, they undergo a neutralization reaction to form ammonium hydroxide and sodium chloride. The chemical equation for this reaction is:
NH4Cl + NaOH → NH4OH + NaCl
In this equation, NH4Cl represents ammonium chloride, NaOH represents sodium hydroxide, NH4OH represents ammonium hydroxide, and NaCl represents sodium chloride.
Learn more about reaction:https://brainly.com/question/28984750
#SPJ1

how could plants be descibed
Answers
Plants can be defined as multicellular organisms that has the ability to manufacture or produce their own food.
What are multicellular organisms?The multicellular organisms are those organisms that are made up of various cells which work together to maintain the functionality of the living organism.
The plant can be described as a multicellular organism as it contains cells such as:
collenchyma, sclerenchyma, parenchyma, xylem and phloem.The plants are also has the ability to manufacture their own food due to the presence of the green pigment called the chloroplast.
Learn more about plants here:
https://brainly.com/question/26557284
#SPJ1
Where are each part of the atoms located?
Answers
Answer: With the portion of hydrogen, all atoms have three parts. The parts of an atom are protons, electrons, and neutrons. A proton is accurately charged and is located in the center or nucleus of the atom. Electrons are negatively charged and are located in rings or orbits spinning around the nucleus.
Explanation: I hope this was helpful.
PLZZZZZZ HEEEELLLLPPPPP!!!!!!!!!!!!!!
What is the relationship between the length of a lever and the force required? (where should the fulcrum be to get the best advantage?)
Answers
Answer:
The answer should be fulcrum
Explanation:
It would be easier if you game me choices to pick from
Condensed structural formulas of glycine and serine Condensed structural formulas of dipeptides Condensed structural formulas of the reactants and products for the hydrolysis of serylglycine Condensed structural formula for the tripeptide
Answers
Condensed structural formulas are written in a single line to save space and make them easier and faster to type out. They display the atoms' positions similarly to a structural formula.
What is the Condensed structural formula of glycine and serine ?The only amino acid that has one carbon atom is glycine. \($-\mathrm{COOH}$\) plus one \($-\mathrm{NH}_2$\) group that is connected to an alpha-carbon and contains two hydrogens. - \($\mathrm{COOH}$\) Serine is made up of an a-carbon, one hydrogen, and one \($-\mathrm{NH}_2$\) group as well as one \($-\mathrm{CH}_2 \mathrm{OH}$\)group.
Both glycylserine and serylglycine are dipeptide molecules made up of glycine and serine; however, glycylserine has an amide linkage formed when one H of the \($-\mathrm{NH}_2$\) group of glycine condenses with the - \($\mathrm{OH}$\) of the - \($\mathrm{COOH}$\) group of glycine Comparably, in serylglycine, one -OH of the - \($\mathrm{COOH}$\) group of serine condenses with one H of the \($-\mathrm{NH}_2$\) group of serine. Serine and glycine are the two byproducts of the hydrolysis of serylglycine. The rupture of the amide bond is seen by the red line. The glycine, serine, and alanine amino acids that make up this tripeptide structure are connected by amide bonds.
To learn more about Condensed structural refer to :
https://brainly.com/question/24708859
#SPJ4
Helllpppp! this is a study island and im stuck :(

Answers
It is clear from the tabular data that, the monkeys in troop 2 were better to avoid predators and more of them were able to reproduce. Hence, option D is correct.
What is natural survival ?In the biosphere, not all living things are fit to survive for a longer life time. Most of them are pray of other higher level animals. Some organisms adopt some strategies to hide from their predators and they can survive more.
It is clear from the table that, the monkeys residing in the ground are more prone to the attacks by their predators. Whereas monkeys living in trees can survive more.
In each year the survival rate is increasing for both troop. However the more number of monkeys which can sustain their population is in troop 2. Therefore, option D is correct.
Find more on natural survival :
https://brainly.com/question/26098341
#SPJ1
a sample of gas weighs 3.33 g and occupies a volume of 1.365 l at 95 °c and 790 torr. identify the gas sample.A. Cl₂ (molar mass-70.90 g/mol)B. NH (molar mass- 17.03 g/mol)C. N₂0 molar mass-44.02 g/mol)D. CHC, (molar mass-119.4 g/mol)E. SO₂ (molar mass - 64.07 g/mol)
Answers
The gas sample is Cl₂. Answer A.
The ideal gas equation formula PV = nRT
P = the gas pressure (atm)1 atm = 760 torr
P = 790 torr = 790 ÷ 760 = 1.04 atmV = the gas volume (L)
V = 1.365 Ln = the number of moles (mol)R = the gas constant = 0.0821 L atm/K molT = the temperature (K)
T = 95 °C = 95 + 273 = 368 K
Calculating the number of moles of the gas sample.
PV = nRT
1.04 × 1.365 = n × 0.0821 × 368
1.4196 = n × 30.21
n = 1.4196 ÷ 30.21
n = 0.04699 mol
The formula for mass and number of moles m = n × Mr
m = the mass of the gas (grams)m = 3.33 gMr = the molar mass (g/mol)
A. Mr Cl₂ = 70.90 g/mol
B. Mr NH₃ = 17.03 g/mol
C. Mr N₂O = 44.02 g/mol
D. Mr CHCl₃ = 119.4 g/mol
E. Mr SO₂ = 64.07 g/mol
Calculating the molar gas from the sample
Mr = m ÷ n
Mr = 3.33 ÷ 0.04699
Mr = 70.9 g/mol
From the info given, gas Cl₂ has the same molar mass as the sample.
So, the gas sample is Cl₂, chlorine gas.
Learn more about ideal gas equation here: https://brainly.com/question/20348074
#SPJ4
help i could use all the help and will be posting more questions later

Answers
you need to add 9L of water to 10mL of the solution of HCl with a pH of 3 to change the pH of 6.
First, calculate the amount of HCl present in 10mL of the solution with a pH of 3:
10mL x 0.1M = 1 mmol HCl
Then, calculate the amount of HCl required to raise the pH to 6:
1 mmol HCl x (10^3 - 10^6) = 9 mmol HCl
Finally, calculate the amount of water required to add 9 m mol of HCl to the solution:
9 mmol HCl x (1L/1000 mmol HCl) = 9L water
Hence, you need to add 9L of water to 10mL of the solution of HCl with a pH of 3 to change the pH of 6.
What is ph?
pH is a measure of acidity and alkalinity. It is measured on a scale of 0 to 14, with 0 being the most acidic and 14 being the most basic (alkaline). A pH of 7 is neutral.
Therefore, you need to add 9L of water to 10mL of the solution of HCl with a pH of 3 to change the pH of 6.
To learn more about ph
Here: https://brainly.com/question/12609985
#SPJ1
Choose from the following.
IRON IS A ELEMENT, COMPOUND, OR MIXTURE?
Answers
Answer:
Iron is element
Explanation:
Answer: element
Explanation: Iron is an element in the periodic table. Nowadays, itis one of the most common elements we use today. The answer is element because iron is an element. It has electrons and protons and atoms.
Hope this helps you!
1.
What is the percent composition of PO4?
Answers
Answer :Phosphorus P 32.6138
Oxygen O 67.3862
Explanation: Phosphorus P 32.6138
Oxygen O 67.3862
what's the answerrr?? :)
Magnesium ribbon reacts with dilute hydrochloric acid. Explain how altering the concentration of the hydrochloric acid alters the rate of the reaction???? (3 marks)
Answers
Explanation:
The reaction between magnesium ribbon and dilute hydrochloric acid is a classic example of a single replacement reaction, which can be represented by the following chemical equation:
Mg (s) + 2HCl (aq) → MgCl2 (aq) + H2 (g)
In this reaction, magnesium (Mg) reacts with hydrochloric acid (HCl) to form magnesium chloride (MgCl2) and hydrogen gas (H2).
The rate of this reaction can be altered by changing the concentration of the hydrochloric acid. This is because the rate of a chemical reaction depends on the concentration of the reactants. Specifically, the rate of the reaction is proportional to the concentration of the reactants raised to some power, which is determined by the reaction's rate law.
In this reaction, the rate law can be expressed as:
Rate = k [Mg] [HCl]^x
Where k is the rate constant and x is the order of the reaction with respect to hydrochloric acid. The order of the reaction with respect to magnesium is one, since the concentration of magnesium does not change during the reaction.
When the concentration of hydrochloric acid is increased, the rate of the reaction increases because there are more hydrochloric acid molecules available to collide with magnesium atoms and react. This means that the value of x is greater than zero and the reaction is dependent on the concentration of hydrochloric acid.
Conversely, when the concentration of hydrochloric acid is decreased, the rate of the reaction decreases because there are fewer hydrochloric acid molecules available to react with magnesium. This means that the value of x is less than one and the reaction is not entirely dependent on the concentration of hydrochloric acid.
Rank these transition metal ions in order of decreasing number of unpaired electrons. If two ions have the same number of unpaired electrons
Fe^3 , Mn^4+ , V3+ , Ni^2+ , Cu^+
Answers
Answer: \(Fe^{3+} > Mn^{4+}\) > \(V^{3+}\) = \(Ni^{2+}\) > \(Cu^+\)
Explanation:
Electronic configuration represents the total number of electrons that a neutral element contains. We add all the superscripts to know the number of electrons in an atom.
Fe: 26: \(1s^2s^22p^63s^23p^64s^23d^{6}\)
\(Fe^{3+}:23:1s^2s^22p^63s^23p^63d^{5}\) : 5 unpaired electrons
Mn: 25: \(1s^2s^22p^63s^23p^64s^23d^{5}\)
\(Mn^{4+}:23:1s^2s^22p^63s^23p^63d^{3}\) : 3 unpaired electrons
V: 23: \(1s^2s^22p^63s^23p^64s^23d^{3}\)
\(V^{3+}:23:1s^2s^22p^63s^23p^63d^{2}\) : 2 unpaired electrons
Ni : 28 : \(1s^2s^22p^63s^23p^64s^23d^{8}\)
\(Ni^{2+}:23:1s^2s^22p^63s^23p^63d^{8}\): 2 unpiared electrons
Cu : 29 : \(1s^2s^22p^63s^23p^64s^13d^{10}\)
\(Cu^{+}:23:1s^2s^22p^63s^23p^63d^{10}\): 0 unpaired electrons
Thus the order of decreasing number of unpaired electrons:
\(Fe^{3+} > Mn^{4+}\) > \(V^{3+}\) = \(Ni^{2+}\) > \(Cu^+\)
in a hydrogen atom, the nucleus (a single proton) has charge e while the single orbiting electron has charge –e. the distance between them is called the "bohr radius". look up the value of the bohr radius and convert it to pm, rb
Answers
The Bohr radius is a fundamental constant in atomic physics that represents the average distance between the nucleus and the electron in a hydrogen atom. The value of the Bohr radius is approximately 0.529 Å (angstroms) or 52.9 pm (picometers).
The Bohr radius (a₀) is a physical constant derived from the Bohr model of the hydrogen atom. It represents the average distance between the nucleus (a single proton) and the orbiting electron in a hydrogen atom. The Bohr model assumes that the electron moves in circular orbits around the nucleus, and the radius of the orbit depends on the energy level of the electron.
The value of the Bohr radius is approximately 0.529 Å (angstroms) or 52.9 pm (picometers). This value is commonly used as a unit to measure atomic sizes and distances. The picometer (pm) is a convenient unit for expressing atomic scales, as it is equivalent to 10^(-12) meters.
The Bohr radius provides a scale for understanding the size of atoms and their electronic structure. It is particularly relevant in hydrogen-like systems, where the electronic structure is similar to that of a hydrogen atom with a single electron orbiting a central nucleus.
The Bohr radius allows scientists to estimate the average size of atomic orbitals and the distances between atoms in molecules.
To know more about electron , click here-
brainly.com/question/371590
#SPJ11
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8