Answers
One dose of antiacid A will neutralize more stomach acid (0.019 mol) than one dose of antacid B (0.01 mol).
Stoichiometric problemStomach acid is HCl. The reaction of HCl with each of the active ingredients of the antiacids is expressed in the following equations:
\(3HCl + Al(OH)_3 --- > AlCl_3 + 3H_2O\).......................Eqn 1
\(2HCl +CaCO_3 --- > CaCl_2 + H_2O + CO_2\)...................Eqn 2
In equation 1, the mole ratio of stomach acid to \(Al(OH)_3\) is 3:1.
Mole of 0.50 g \(Al(OH)_3\) = 0.5/78 = 0.0064 mol
The equivalent amount of stomach acid to be neutralized = 0.0064 x 3 = 0.0192 mol
In equation 2, the mole ratio of stomach acid to \(CaCO_3\) is 2:1.
Mole of 0.50 g \(CaCO_3\) = 0.5/100 = 0.005
The equivalent amount of stomach acid to be neutralized = 0.005 x 2 = 0.01 mol.
Thus, one dose of antiacid A will neutralize more stomach acid (0.019 mol) than one dose of antacid B (0.01 mol).
More on stoichiometric problems can be found here: https://brainly.com/question/23742235
#SPJ1
Related Questions
PLEASE HELP WITH PAGE 1 FOR QUESTIONS 1, 2, 3 WITH THE GRAPHING THANK YOU.
Answers
The density of gold is 19.3 g/cm3. What is this density in the units of lbs/in3? (1 in = 2.54 cm and 1 lb = 453.6 g).
Answers
Answer:
\(\rho =0.697\frac{lb}{in^3}\)
Explanation:
Hello,
In this case, given that 1 inch equals 2.54 cm and 1 lb equals 453.6 g we apply the following conversion factor in order to compute the required density in lb/in³:
\(\rho=19.3\frac{g}{cm^3}*\frac{1lb}{453.6g} *(\frac{2.54cm}{1in} )^3\\\\\rho =0.697\frac{lb}{in^3}\)
Best regards.
Help me please I need the answer as soon as possible

Answers
Answer:
see in your book properly
Charge of calcium atom
Answers
Answer: The calcium ion has a 2+ charge and the phosphate ion has a 3- charge.
Strontium hydroxide reacts with hydrobromic acid to produce Strontium bromide and
water.
Write and balance the chemical reaction above, use it for problems 1-4 below:
1. If 5.50 moles of strontium hydroxide were consumed, how much moles of water are
produced?
2. Find the mass of hydrobromic acid used to produce 7.50 moles water.
3. If 10.8 g of strontium hydroxide were used, how much moles of strontium bromide are
produced?
4. If 13.3 g of hydrobromic acid were consumed, find the mass of the water produced.
Answers
Sr(OH)2 + 2 HCl --> SrCl2 + 2 H2O
To find the moles of water produced when 5.50 moles of strontium hydroxide are consumed, we need to apply the law of conservation of mass. The mass of water produced is equal to the mass of strontium hydroxide consumed. Since strontium hydroxide has a molar mass of 142 g/mol and water has a molar mass of 18 g/mol, 1 mol of strontium hydroxide can produce 9 mol of water. Therefore, 5.50 moles of strontium hydroxide can produce 49.5 mol of water.
Similarly, 7.50 moles of water can be produced by reacting 18 moles of hydrobromic acid with strontium hydroxide. Hydrobromic acid has a molar mass of 79.9 g/mol, so 18 moles of hydrobromic acid would have a mass of 79.9 * 18 = 1435.2 g.
To find the moles of strontium bromide produced when 10.8 g of strontium hydroxide is used, we need to apply the law of conservation of mass again. The mass of the strontium bromide produced is equal to the mass of strontium hydroxide consumed. Since strontium bromide has a molar mass of 410 g/mol and strontium hydroxide has a molar mass of 142 g/mol, 1 mol of strontium bromide can consume 3.23 moles of strontium hydroxide. Therefore, 10.8 g of strontium hydroxide can produce 10.8 / 3.23 = 3.34 moles of strontium bromide.
Finally, to find the mass of water produced when 13.3 g of hydrobromic acid is consumed, we need to apply the law of conservation of mass yet again. The mass of the water produced is equal to the mass of hydrobromic acid consumed. Since hydrobromic acid has a molar mass of 79.9 g/mol, 13.3 g of hydrobromic acid would produce 13.3 / 79.9 = 0.166 moles of water.
Butane gas (C4H10) burns in oxygen gas to produce carbon dioxide gas and water vapor. Balance the equation for this reaction (in lowest multiple integers). Write the unbalanced equation for this reaction (listed in the same order as given in the problem).
Answers
Answer:
C₄H₁₀(g) + O₂(g) ⇒ CO₂(g) + H₂O(g)
2 C₄H₁₀(g) + 13 O₂(g) ⇒ 8 CO₂(g) + 10 H₂O(g)
Explanation:
Butane gas (C₄H₁₀) burns in oxygen gas to produce carbon dioxide gas and water vapor. The unbalanced equation is:
C₄H₁₀(g) + O₂(g) ⇒ CO₂(g) + H₂O(g)
First, we will balance carbon and hydrogen which are in just one compound on each side.
C₄H₁₀(g) + O₂(g) ⇒ 4 CO₂(g) + 5 H₂O(g)
Finally, we will balance the oxygen atoms.
C₄H₁₀(g) + 6.5 O₂(g) ⇒ 4 CO₂(g) + 5 H₂O(g)
In order to have integers, we will multiply everý compound by 2.
2 C₄H₁₀(g) + 13 O₂(g) ⇒ 8 CO₂(g) + 10 H₂O(g)
1.0 mole of a gas is enclosed in a 12.3 liter cylinder with a moveable piston at 300 K and 2.0 atm. Half of the gas is removed, leaving 0.50 mole in the cylinder and the system is warmed to 900 K. The cylinder changes volume to maintain constant pressure. What is the volume in the final system?1.0 mole of a gas is enclosed in a 12.3 liter cylinder with a moveable piston at 300 K and 2.0 atm. Half of the gas is removed, leaving 0.50 mole in the cylinder and the system is warmed to 900 K. The cylinder changes volume to maintain constant pressure. What is the volume in the final system?
Answers
The final volume of the system, given that half of the gas is removed, leaving 0.50 mole in the cylinder and the system is warmed to 900 K is 18.45 liters
How do I determine the final volume of the system?From ideal gas equation, we have
PV = nRT
Rearrange
V / nT = R/ P
R / P = Constant
Thus, we have
V₁ / n₁T₁ = V₂ / n₂T₂
Where
V₁ and V₂ are initial and final volumen₁ and ₂ are initial and final moleT₁ and T₂ are initial and final temperatureWith the above formula, we can obtain the final volume of the system as follow:
Initial mole (n₁) = 1 moleInitial volume of gas (V₁) = 12.3 litersInitial temperature (T₁) = 300 KPressure = ConstantFinal mole (n₂) = 0.5 moleFinal temperature (T₂) = 900 KFinal volume (V₂) = ?V₁ / n₁T₁ = V₂ / n₂T₂
12.3 / (1 × 300) = V₂ / (0.5 × 900)
Cross multiply
1 × 300 × V₂ = 12.3 × 0.5 × 900
300 × V₂ = 5535
Divide both side by 300
V₂ = 5535 / 300
V₂ = 18.45 liters
Thus, the final volume of the system is 18.45 liters
Learn more about volume:
https://brainly.com/question/14560487
#SPJ1
Please help meeeeeee ? what is the answer

Answers
Answer:
D
Explanation:
In terms of relative energy, gas particles have the most energy, solid particles have the least energy and liquid particles are somewhere in between.
What is the most strongest material bedrock Obsidian or diamond
Answers
Select the true statements about SDS‑PAGE, a method of separating proteins. Assume that SDS‑PAGE is performed under reducing conditions.
a. Proteins are separated in a polyacrylamide gel matrix.
b. Protein‑SDS complexes have similar mass to charge ratios; therefore, separation is by size.
c. Proteins are visualized using a dye that binds to the gel matrix, but not to proteins.
d. Smaller proteins migrate faster through the polyacrylamide gel.
e. Sodium dodecyl sulfate binds proteins, resulting in protein‑SDS complexes that are similar in size.
f. Protein‑SDS complexes migrate toward the negative electrode.
Answers
Helppppp pleaseeee xxxxxx
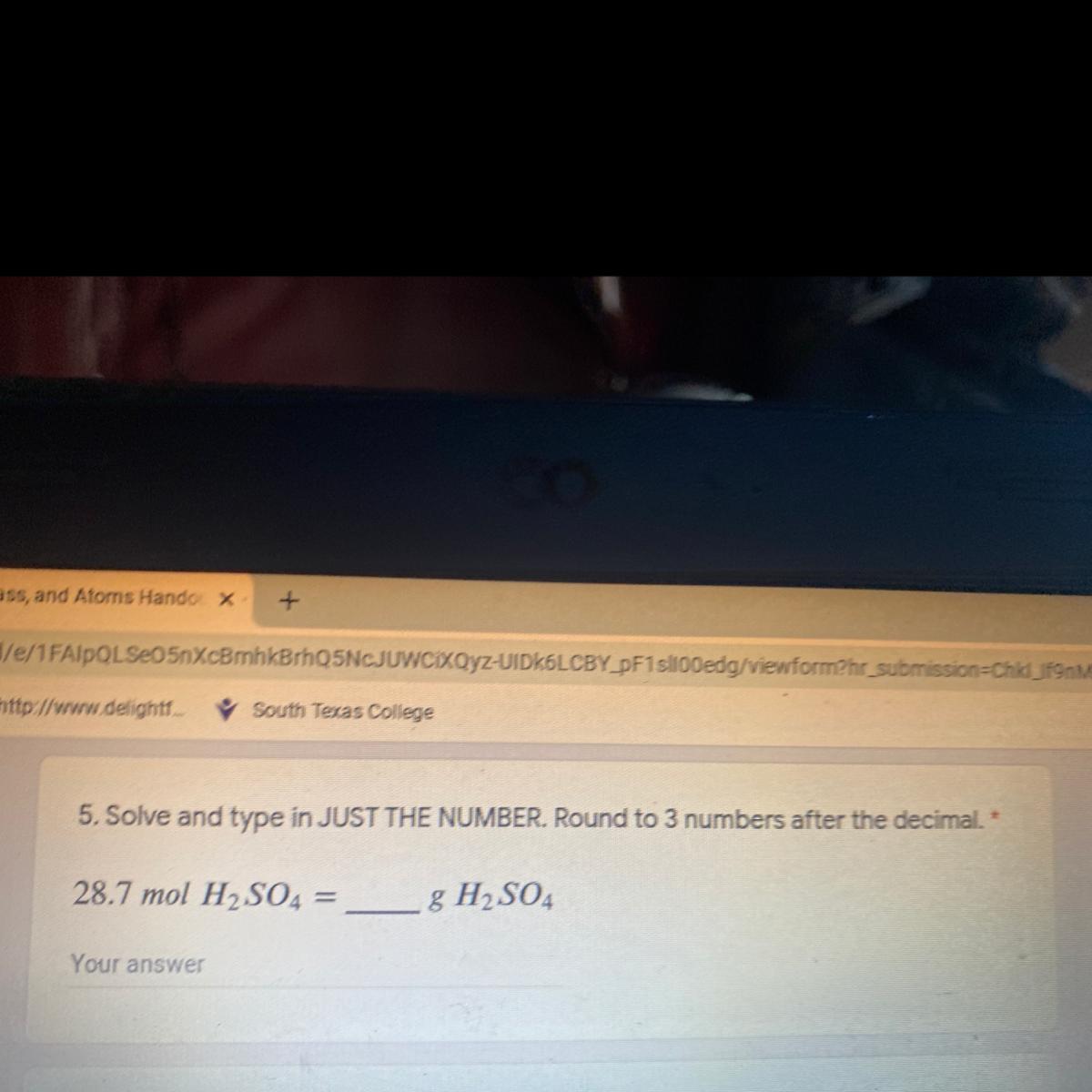
Answers
Answer:
2812.6 g of H₂SO₄
Explanation:
From the question given above, the following data were obtained:
Mole of H₂SO₄ = 28.7 moles
Mass of H₂SO₄ =?
Next, we shall determine the molar mass of H₂SO₄. This can be obtained as follow:
Molar mass of H₂SO₄ = (1×2) + 32 + (16×4)
= 2 + 32 + 64
= 98 g/mol
Finally, we shall determine the mass of H₂SO₄. This can be obtained as follow:
Mole of H₂SO₄ = 28.7 moles
Molar mass of H₂SO₄ =
Mass of H₂SO₄ =?
Mole = mass / Molar mass
28.7 = Mass of H₂SO₄ / 98
Cross multiply
Mass of H₂SO₄ = 28.7 × 98
Mass of H₂SO₄ = 2812.6 g
Thus, 28.7 mole of H₂SO₄ is equivalent to 2812.6 g of H₂SO₄
Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
Which group on the periodic table has the most reactive metals?
A.
group 1
B.
group 3
C.
group 18
D.
group 17
Answers
Answer:
A group 1
Explanation:
Group 1
reactivity decreases as you go from L to R or downward on the periodic table
1
Aluminum metal and oxygen gas combine to produce aluminum oxide.
Which of these is the balanced equation for this reaction? *
Answers
Answer:
4 Al(s) + 3 O₂(g) ⇒ 2 Al₂O₃(s)
Explanation:
Let's consider the unbalanced molecular equation when aluminum metal and oxygen gas combine to produce aluminum oxide. This is a combination reaction.
Al(s) + O₂(g) ⇒ Al₂O₃(s)
We can balance O atoms by multiplying O₂ by 3 and Al₂O₃ by 2.
Al(s) + 3 O₂(g) ⇒ 2 Al₂O₃(s)
Finally, we multiply Al by 4 to get the balanced equation.
4 Al(s) + 3 O₂(g) ⇒ 2 Al₂O₃(s)
I need help with question 4. The problem is asking to match the reactions with the reaction types:acid/base, precipitation or redox.

Answers
According to our reactions:
1) Li(s) + O2(g) ==> Li2O(s)
It is a Redox reaction, Li is oxidized and O is reduced. We have here loss and gain of electrons.
2) It is a precipitation reaction because when the PbI2 is formed, it precipitates.
3) This is the reaction of an acid with a base, so it is an acid/base reaction.
Which chapter is bulk properties of matter?
Answers
The list which includes only bulk properties of matter is boiling point, surface tension and vapor pressure.
Definition of matter
Something that has mass and occupies space is defined as a matter.
Hence, all the physical objects or substances that are found on the planet Earth are typically composed of matter.
Matter are classified into three (3) states:
Gas
Solid
Liquid
The property of the matter that affects matter as a whole is known as bulk property of matter and they are listed as follows:
Boiling pointElasticityStrainStressSurface tensionVapor pressureHence, boiling point, vapor pressure and surface tension are considered as a bulk properties of matter.
Learn more about bulk properties of matter from the link given below.
https://brainly.com/question/29546212
#SPJ4
Based on the molecular views, classify each substance as an atomic element, a molecular element, an ionic compound, or a molecular compound.
Answers
A molecular compound is defined as a molecule whose formula states the actual number of atoms bonded together in the molecule.
The atoms are joined to give a definite shape which is defined by the angles between the bonds and by the bond lengths. Molecular compounds can contain discrete molecules, which are held together by sharing electrons in covalent bonding.
Examples of molecular compounds are water, which contains H2O molecules; methane contains CH4 molecules; and hydrogen fluoride, which contains HF molecules.
As a rule of thumb, compounds that are involved in a metal binding with either a non-metal or a semi-metal will display ionic bonding.
Compounds can be composed with only non-metals or semi-metals and non-metals will display covalent bonding and will be classified as molecular compounds.
To know more about molecular compounds:
https://brainly.com/question/18158405
#SPJ4
During a UV-Visible spectroscopy experiment, a student notes a wavelength peak measurement at 280 nm. What region of the electromagnetic spectrum was this peak observed?
Answers
If during a spectroscopy experiment a student notes a wavelength peak measurement at 280 nm, then the region of the electromagnetic spectrum observed is the UV region.
What is the electromagnetic spectrum?The electromagnetic spectrum refers to all types of wavelengths that radiation can emit as light, which involves both visible light and also ultraviolet (UV) radiation.
In conclusion, if during a spectroscopy experiment a student notes a wavelength peak measurement at 280 nm, the region of the electromagnetic spectrum observed is the UV region.
Learn more about the electromagnetic spectrum here:
https://brainly.com/question/23423065
#SPJ1
A swimming pool, 10.0 m by 4.0 m, is filled with water to a depth of 3.0 m at a temperature of 20.2°C.
If the energy needed to raise the temperature of the water to 27.3°C is obtained from the combustion of methane (CH4), what volume of methane, measured at STP,
must be burned?
AH combustion for CH4 = -891 kJ/mol
volume CH4 needed =
Answers
First, we need to determine the mass of water in the pool:
mass = density x volume
density of water = 1000 kg/m³
volume = length x width x depth
volume = 10.0 m x 4.0 m x 3.0 m = 120 m³
mass = 1000 kg/m³ x 120 m³ = 120000 kg
Next, we need to calculate the heat required to raise the temperature of the water:
q = m x c x ΔT
where q is the heat energy, m is the mass of water, c is the specific heat of water, and ΔT is the change in temperature.
c = 4.18 J/g°C (specific heat of water)
ΔT = 27.3°C - 20.2°C = 7.1°C
m = 120000 kg
q = 120000 kg x 4.18 J/g°C x 7.1°C = 35792400 J
Next, we need to convert the energy required to burn methane to heat energy:
-891 kJ/mol x (1 mol CH4/160 g CH4) x (1000 g/1 kg) = -5.569 kJ/g
We can now calculate the amount of methane needed:
energy = -5.569 kJ/g x mass CH4
mass CH4 = energy / (-5.569 kJ/g)
mass CH4 = 35792400 J / (-5569 J/g) = -6431.6 g
At STP, 1 mole of any gas occupies 22.4 L of volume. We can use this to convert the mass of methane to volume at STP:
1 mol CH4 = 16 g CH4
-6431.6 g CH4 x (1 mol CH4/16 g CH4) x (22.4 L/1 mol CH4) = -9074.4 L
Since we cannot have a negative volume, we can take the absolute value of the result:
|9074.4 L| = 9074 L
Therefore, approximately 9074 liters of methane gas at STP must be burned to raise the temperature of the water in the pool from 20.2°C to 27.3°C.
A voltaic electrochemical cell is constructed using the following reaction. The half-cell components are separated by a salt bridge. Br2(l) + Mg(s) >2Br(aq) +Mg2*(aq) Write the reactions that take place at the anode and at the cathode, the direction in which the electrons migrate in the external circuit, and the direction the anions in the salt bridge migrate. Use smallest possible integer coefficients If a box is not needed, leave it blank Enter the reaction that takes place at the anode. Include state symbols Enter the reaction that takes place at the cathode. Include state symbols: 7+ In the external circuit, electrons migrate the Mg electrode ▼ the Br2 electrode ▼ Anions migratethe salt bridgethe Br2 compartment.
Answers
In the salt bridge, anions migrate from the F-|F2
The anode reaction is :
2I^-(aq) -------> I2(g) +2e
Cathode reaction
F2(g) + 2e------> 2F^-(aq)
In the external circuit, electrons migrate from the I-|I2 electrode (anode) to the F-|F2 electrode (cathode)
In the salt bridge, anions migrate from the F-|F2
Oxidation occurs at the anode while reduction occurs at the cathode. Anode is a positive electrode in this case, whereas cathode is a negative electrode. Oxidation occurs at the anode while reduction occurs at the cathode. The species that are oxidising provide the electrons. Any electrode where oxidation occurs is an anode. Water electrolysis is one straightforward example. The anode is a platinum electrode that is positively charged and used to convert H2 gas into H+ ions.
To learn more about anode reaction visit:https://brainly.com/question/27982574
#SPJ4
5. Neon has two major isotopes. Neon-20 and Neon-22. Out of every 250 neon atoms, 225 will be Neon-20
(19.992 g/mol), and 25 will be Neon-22 (21.991 g/mol). What is the average atomic mass of Neon?
Answers
Answer:
20.1974
Explanation:
The Atomic mass will be the same for every isotope as the number of Neutrons changes, not the number of protons.
Neon has two major isotopes. Neon-20 and Neon-22. Out of every 250 neon atoms, 225 will be Neon-2(19.992 g/mol), and 25 will be Neon-22 (21.991 g/mol), then the average atomic mass of Neon20.1974
What are the difference between atomic number and atomic mass ?Atomic mass of an element can be defined as the total number of neutrons and protons present in the nucleus of an atom and the atomic number is the number of protons present in nucleus.
the average weight of an element is called as atomic mass while atomic number is total number of protons present in the central nucleus of the atom. Atomic mass is presented a symbolic representation of A where as atomic number is represented by the letter Z.
Atomic mass can not classified which type of element where as Atomic number classify and identify the type of element, there are different types of isotopes of an element observed on the basis of their atomic mass where as isotopes only share the same atomic number.
Neon has two isotopes such as , N e 20, N e 21 and N e 22.The average atomic mass can be calculated as:
N e Average atomic mass = [ 19.9924 (90.92 %)] + [ 20.9940 (0.257 %)] + [ 21.9914 (8.82 %)] a m u
Learn more about atomic mass, here:
https://brainly.com/question/17067547
#SPJ2
4. Round off the following results to three significant figures:
a) 23.01 g
Answers
the answer should be a the the question
'Which of the following best describes cis-trans isomers? See Concept 4.2 (Page 60)View Available Hint(s)They differ in the arrangement of covalent bonds and in covalent partners.They are long chains of hydrogen and carbon atoms.They are mirror images of each other.They have the same number of atoms of the same elements but different structures.They differ in their spatial arrangement around inflexible double bonds
Answers
The best description of cis-trans isomers is that they differ in their spatial arrangement around inflexible double bonds. Option E is correct.
Specifically, cis-trans isomers are a type of stereoisomers that have the same molecular formula and the same covalent bonds but differ in the spatial arrangement of their atoms due to the inflexibility of a double bond. In cis isomers, the two substituents on each carbon atom are on the same side of the double bond, while in trans isomers, the two substituents on each carbon atom are on opposite sides of the double bond.
This results in different physical and chemical properties for the two isomers. For example, cis and trans isomers of some compounds may have different boiling points, melting points, and reactivities. Cis-trans isomerism is an important concept in organic chemistry as it affects the properties and behavior of molecules and can have significant implications for their biological activity, environmental fate, and industrial applications. Option E is correct.
To know more about the Covalent bonds, here
https://brainly.com/question/13130950
#SPJ1
if you know any answer to this pls help out I'll give brainiest plus a lot of points

Answers
Answer:
I cant see it clearly
Explanation:
:(
What are specific groups of other atoms attached to a hydrocarbon chain called?
add-ons
functional groups
organic extras
tails
Answers
The specific groups of other atoms attached to a hydrocarbon chain are called functional groups.
These are specific atoms or groups of atoms that are attached to the hydrocarbon chain and give the molecule its characteristic chemical and physical properties.
Functional groups include, for example, hydroxyl (-OH), carbonyl (C=O), amino (-NH₂), and carboxyl (-COOH) groups, among others.
They are the reactive part of the molecule and determine the molecule's reactivity and its interactions with other molecules.
Thus, option B is correct.
To learn more about the functional group, follow the link:
https://brainly.com/question/31522322
#SPJ1
What is the theoretical yield, in grams, of CO₂, if 29.9 g of C₂ H₂ completely reacts?
Answers
The theoretical yield, in grams, of CO₂ is 93.97g having molar mass 44g
What is molar mass ?
The "counting unit" used by chemists to describe the quantity of atoms, ions, molecules, or formula units in a specific chemical sample is called a mole. The mole is comparable to other counting units you have employed in the past, such as pair (2), dozen (12), and gross (144). Avogadro's number (6.022 x 1023) of molecules (or formula units) make up one mole of a substance (ionic compound). The mass of 1 mole of a chemical is indicated by its molar mass. It provides you with the amount of grammes per mole of a substance, to put it another way. Therefore, grams/mole are the units for molar mass.
2C2H2 + 5O2=====> 4CO2+2H2O
Molar Mass of C2H2 = 2*12+2*2 = 28g = 1 mol
2 mol of C2H2 = 28*2 = 56g
Molar Mass of CO2 = 12+2*16 = 44g = 1 mol
4 mol of CO2 = 4*44 = 176g
56g of C2H2 gives 176g of CO2
29.9g of C2H2 gives 176*29.9/56g of CO2
= 93.97g of CO2
The theoretical yield, in grams, of CO₂ is 93.97g having molar mass 44g
To know more about molar mass. from the given link
https://brainly.com/question/837939
#SPJ9
Zircons from the basalt flow we’re measured to have 95.8% uranium-238, and 4.2% Lead-206. What is the age of the basalt flow?
Answers
Answer:
5
Explanation:
A sample of 0.49 g of carbon dioxide was obtained by heating 1.22 g of calcium carbonate. What is the percent yield for this reaction
Answers
The percent yield for the reaction of a sample of 0.49 g of carbon dioxide was obtained by heating 1.22 g of calcium carbonate is 92.4%
Given the mass of carbon dioxide (\(CO2\)) = 0.49g
The mass of calcium carbonate (\(CaCO3\)) = 1.22g
The reaction is as follows:
\(CaCO3(s) -- > CaO(s)+CO2(s)\)
As we see 1 mole of CaCO3 is required to produce 1 mole of \(CO2\)
The molar mass of calcium carbonate, = 100.09 g/mol.
The molar mass of given carbon dioxide = 44g
mass of \(CaCO3\) used = number of moles x molar mass = 1 * 100 = 100g
Mass of \(CO2\) produced = 1 * 44 = 44g
Here for 100g of \(CaCO3\) 44g of \(CO2\) is produced.
Then for 1.22g of \(CaCO3\) = 44 * 1.22/100 = 0.53g of \(CO2\) is produced.
But the actual yield of carbon dioxide is 0.49 g
The percent yield is calculated by dividing the actual yield by the theoretical yield and multiplying by 100.
percent yield = 0.49/0.53 * 100 = 92.4%
To learn more about moles click here https://brainly.com/question/26416088
#SPJ1
What is the best method of separating the mixture of sand and fine salt?
Answers
By using filtration, the sand and fine salt can be effectively separated based on their difference in particle size, providing a clean separation of the two components.
Filtration is a separation technique that takes advantage of the difference in particle size between sand and salt. It involves passing the mixture through a porous material, such as filter paper or a filter funnel, which allows the liquid (saltwater) and small salt particles to pass through while retaining the larger sand particles.
Here's how the filtration process can be carried out:
1. Set up a filter apparatus with a funnel and filter paper or a filter flask.
2. Place the mixture of sand and salt in a beaker or a flask.
3. Slowly pour the mixture into the filter paper or funnel, allowing the liquid (saltwater) to pass through while retaining the sand on the filter paper.
4. Once the liquid has passed through completely, the sand will be left behind on the filter paper or in the filter flask.
5. Carefully remove the sand from the filter paper or filter flask, and the saltwater solution can be collected separately.
For more such questions on filtration
https://brainly.com/question/29756050
#SPJ8
Limestone is used in industry as a raw material in the production of slaked Ca (OH) 2.
1) Write the equations of the chemical reactions that characterize the transformations carried out in the production process!
CaCO3 -> CaO -> Ca (OH) 2
The first reaction equation is how calcium oxide can be obtained from calcium carbonate.
The second reaction equation - how calcium oxide can be obtained from calcium oxide.
Answers
Answer:
i k ow its not the exact anwser but ih ope this helps
Explanation:
Energy plays a key role in chemical processes. According to the modern view of chemical reactions, bonds between atoms in the reactants must be broken, and the atoms or pieces of molecules are reassembled into products by forming new bonds. Energy is absorbed to break bonds, and energy is evolved as bonds are made. In some reactions the energy required to break bonds is larger than the energy evolved on making new bonds, and the net result is the absorption of energy. Such a reaction is said to be endothermic if the energy is in the form of heat. The opposite of endothermic is exothermic; in an exothermic reaction, energy as heat is evolved. The more general terms exoergic (energy evolved) and endoergic (energy required) are used when forms of energy other than heat are involved.
A great many common reactions are exothermic. The formation of compounds from the constituent elements is almost always exothermic. Formation of water from molecular hydrogen and oxygen and the formation of a metal oxide such as calcium oxide (CaO) from calcium metal and oxygen gas are examples. Among widely recognizable exothermic reactions is the combustion of fuels (such as the reaction of methane with oxygen mentioned previously).