The 1h nmr spectrum of a compound which has the molecular formula c6h12o2 is shown below. Draw its structure.
Answers
The 1H NMR spectrum of a compound with the molecular formula C6H12O2 is displayed below. Its structure will be drawn. The NMR spectrum's information can be used to construct the molecule's structure by understanding how protons in various chemical environments give rise to specific signals.
NMR spectroscopy offers a powerful method to investigate the molecular structures of compounds. The signal at 2.05 ppm corresponds to three identical protons (triplet), while the signal at 1.25 ppm corresponds to two identical protons (quintet).
The presence of a triplet and a quintet in a 3:2 ratio indicates that the molecule has two types of protons that are near to each other and are in a ratio of 3:2. To begin, we know that the molecule has six carbon atoms, because the molecular formula is C6H12O2.
To know more about compound visit:
https://brainly.com/question/14138124
#SPJ11
Related Questions
Which element has the highest (most negative) electron affinity?Group of answer choicesA. LiB. KrC. SD. MgE. Cr
Answers
Among the given elements, Chlorine (symbol: Cl) has the highest (most negative) electron affinity.
Electron affinity is defined as the amount of energy released or absorbed when an electron is added to a neutral atom in the gaseous state to form a negative ion. Chlorine has an electron affinity of -349 kJ/mol, which is the highest among the given options.
Electron affinity is a physical property of an atom or a molecule that refers to the amount of energy released or absorbed when an electron is added to a neutral atom or molecule to form a negative ion. It is a measure of how much an atom or a molecule "likes" or attracts an additional electron.
Therefore, the correct answer is C. Cl.
learn more abouT electron affinity here:
https://brainly.com/question/977718
#SPJ4
The density of water is 1.00 g/mL. If your swimming pool holds 208,000 gallons of water, what are the conversion factors that are needed to find the weight of the water in a filled swimming pool in tons?A) 1 ton - 2000 pounds3.7854 gal - 1L1000 L - 1 mL1 pound - 453.59 gB) 1 ton - 2000 pounds3.7854 gal -1 L1000 mL - 1 L1 pound - 453.59 gC) 1 ton - 2000 pounds3.7854 L - 1 gal1000 L - 1 mL1 pound-453.59 gD) 1 ton - 2000 pounds3.7854L - 1 gal1000 mL - 1 L1 pound - 453.59 g
Answers
1) List the known and unknown quantities.
Volume: 208,000 gallons.
Density: 1.00 g/mL.
2) Convert gallons to liters.
3.7854 L = 1 gal.
\(L=208000\text{ }gal*\frac{3.7854\text{ }L}{1\text{ }gal}=787365.651\text{ }L\)3) Convert g/mL to pound/L
1 pound = 453.59 g
1000 mL = 1 L
\(\frac{kg}{L}=1.00\frac{g}{mL}*\frac{1000\text{ }mL}{1\text{ }L}*\frac{1\text{ }pound}{453.59\text{ }g}=2.205\frac{pound}{L}\)4) Mass of water in kg.
\(density=\frac{mass}{volume}\)\(mass=density*volume\)\(mass=2.205\frac{pound}{L}*787365.651\text{ }L=1736141.26\text{ }pounds\)5) Convert pounds to tons.
1 ton = 2000 pounds
\(ton=1736141.26\text{ }pounds*\frac{1\text{ }ton}{2000\text{ }pounds}=868.07\text{ }tons\)The conversion factors needed are those in option D.
.
N_{2}(g) + 3H_{2} * (g) < =2NH 3 (g)+heat What will happen to equilibrium if the temperature decreases?

Answers
ANSWER
The arrow will be shifted to the right (OPTION B)
EXPLANATION:
Firstly, we need to write out the chemical reaction equation
\(N_{2(g)}+3H_{2(g)}\text{ }\rightleftarrows2NH_{3(g)}\text{ + heat}\)From the reaction above, you will see that heat is one of the products, this means that the reaction is n exothermic reaction.
An exothermic reaction is a type of reaction in which heat is released to its surroundings.
In an exothermic reaction, a decrease in temperature will shift the equilibrium to the right
at a certain point in each reaction the solution turned blue, indicating that a ph change had occurred. what causes this ph change?
Answers
Using indicators at a certain point in each reaction the solution turned blue, indicating that a pH change had occurred due to increase in the hydroxide ion concentration.
What is an indicator?Indicator is defined as a chemical substance which is chemically a weak acid or a weak base which changes it's color depending upon the concentration of hydrogen ions present in the solution.They dissociate slightly in water to produce ions.
These are generally derived from plant pigments and are of slightly acidic or basic in nature.There are three types of indicators:
1) natural indicators
2) synthetic indicators
3) olfactory indicators.
These are mainly used in determination of end point of titrations. Every indicator has it's pH range in which it can perform effectively.These are usually organic compounds.
Learn more about indicators,here:
https://brainly.com/question/12708635
#SPJ2
What is the pH of a solution with [H+] concentration of 1.86 x 10-5?
a.5.37 x 10-10
b.9.3
c.4.7
d.1.86 x 10-5
Answers
Answer:
4.7Explanation:
The pH of a solution can be found by using the formula
\(pH = - log [ {H}^{+} ]\)
From the question we have
\(ph = - log(1.8 \times {10}^{ - 5} ) \\ = 4.7447\)
We have the final answer as
4.7Hope this helps you
Are two atoms of the same element identical??
Answers
Using the half-life graph of cobalt-57, choose ALL the statements that are accurate.
es )
A)
The half-life of Co-57 is 20 days.
B)
The half-Life of Co-57 is 10 days.
C)
After fifty days, there is 0% or no Co-57 remaining.
D)
After twenty days have elapsed, about 25% of the Co-57 remains.
E)
Scientists have an 8 gram sample of Co-57. After thirty days, 1 gram
remains.
Answers
Answer: B) The half-life of Co-57 is 10 days
D) After twenty days have elapsed, about 25% of the Co-57 remains.
E) Scientists have an 8 gram sample of Co-57. After thirty days, 1 gram remains.
Explanation:
Do 150.0 grams and 150 grams mean the same thing
Answers
Answer:
yes 150.0grams = 150grams
if 6 moles of a a compound produce 84 J of energy, what is the h reaction in j/mol
Answers
The enthalpy of the reaction is 14 J/mol.
The enthalpy of a reaction (ΔH) is the amount of energy transferred between a system and its surroundings during a chemical reaction at constant pressure, measured in joules per mole (J/mol). This value is important because it can tell us whether a reaction is exothermic or endothermic, as well as give us information about the strength of chemical bonds within the reactants and products.To calculate the enthalpy of a reaction, we need to know the amount of energy released or absorbed (Q) and the number of moles of the compound involved in the reaction (n). We can use the equation:
ΔH = Q/n
Given that 6 moles of a compound produce 84 J of energy, we can calculate the enthalpy of the reaction as follows:
ΔH = Q/n
ΔH = 84 J / 6 mol
ΔH = 14 J/mol
This means that for every mole of the compound involved in the reaction, 14 J of energy is transferred between the system and the surroundings. Since the value is positive, we can conclude that the reaction is endothermic, meaning that it requires an input of energy to occur.It is worth noting that the enthalpy of a reaction can depend on a number of factors, such as temperature, pressure, and the specific conditions under which the reaction occurs. As such, it is important to take these factors into account when calculating or predicting enthalpy values.
for such more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
What is the electron configuration silver, Ag? exeception

Answers
Answer
2,8,18,18,1
Explanation:
(earth science not chem!!) What branch of Earth Science deals with stars and galaxies beyond Earth?
Answers
Answer:
The answer is Astronomy.
Explanation: Astronomy is the study of objects beyond the Earth’s atmosphere
3. Calculate the concentration in g/dm3 of solution of NaOH containing 0.25 mole in 1dm3
Answers
The concentration of the NaOH solution is 10.00 g/dm³.
To calculate the concentration in g/dm³ of a solution of NaOH containing 0.25 moles in 1 dm³, we need to know the molar mass of NaOH.
The molar mass of NaOH is calculated as follows:
Na (Sodium) = 22.99 g/mol
O (Oxygen) = 16.00 g/mol
H (Hydrogen) = 1.01 g/mol
Molar mass of NaOH = 22.99 g/mol + 16.00 g/mol + 1.01 g/mol = 40.00 g/mol
Now, we can calculate the concentration (C) using the formula:
C = (moles of solute) / (volume of solution in dm³)
C = 0.25 moles / 1 dm³
C = 0.25 mol/dm³
To convert moles to grams, we can multiply the molar mass by the number of moles:
Concentration in g/dm³ = (0.25 mol/dm³) * (40.00 g/mol)
Concentration in g/dm³ = 10.00 g/dm³
To know more about Hydrogen
https://brainly.com/question/31018544
#SPJ11
what is mass in grams of 175 mL of salt water with a density of 1.30 g/cm3
Answers
Answer:
Answer: 227.5 grams
Explanation:
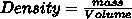
Answer:
227.5
Explanation:
Acellus
The active ingredient in a Tum® antacid tablet i calcium carbonate (CaCO 3, FM = 100. 09), it neutralize exce hydrochloric acid (HCl, FM = 36. 46) in the tomach via the reaction CaCO 3 () 2HCl(aq) → CaCl 2 (aq) H 2 O(l) CO 2 (g). A certain doe of Tum containing 750 mg of CaCO 3 i added to 25 mL of 0. 100 M HCl. What i the volume of CO 2 generated under condition of STP?
Answers
The volume of CO₂ generated under condition of STP in the reaction :
CaCO₃ + 2HCl -----> CaCl₂ + H₂O + CO₂ is 12.5 mL.
The reaction is given as :
CaCO₃ + 2HCl -----> CaCl₂ + H₂O + CO₂
mass of CaCO₃ = 750 mg = 0.75 g
molarity of HCl = 0.100 M
volume of HCl = 25 mL = 0.025 L
moles of CaCO₃ = 0.75 / 100
= 0.0075 mol
moles of HCl = 0.100 × 0.025
= 0.0025 mol
here HCl is limiting reagent , formation of CO₂ depends on HCl
2 moles of HCl = 1 mole of CO₂
0.0025 mol of HCl = 0.0025 / 2
= 0.00125 mol
volume of CO₂ = moles / molarity
= 0.00125 / 0.100
= 0.0125 L = 12.5 mL
To learn more about moles here
https://brainly.com/question/29724957
#SPJ4
what new functional group is formed during an elimination reaction chem 3a berkeley
Answers
During an elimination reaction in organic chemistry, a new double bond (π bond) is formed, resulting in the creation of an alkene functional group. This process involves the removal of a leaving group and the adjacent hydrogen atom from a molecule, resulting in the formation of a double bond between the two adjacent carbon atoms.
In elimination reactions, a strong base or acid is often used to abstract the proton from the adjacent carbon atom, generating a carbanion intermediate. The leaving group is then expelled from the molecule, and the carbanion intermediate undergoes a rearrangement to form a more stable carbocation. Finally, the base or another molecule acts as a nucleophile, capturing a proton from the carbocation to form the double bond. This newly formed double bond represents the alkene functional group and is characteristic of elimination reactions in organic chemistry.
Learn more about Organic chemistry here ; brainly.com/question/14623424
#SPJ11
Calculate the maximum β - energy in the decay of
14
C to
14
N. The mass of
14
C is 14.003241u; the mass of
14
N is 14.003074u
Answers
Question id : 33544103
Answer:
To calculate the maximum β-energy in the decay of carbon-14 (14C) to nitrogen-14 (14N), we need to use the mass difference between the initial and final nuclei. The β-decay process involves the conversion of a neutron into a proton, emitting a β-particle (electron) and an electron antineutrino.
The mass difference (∆m) between 14C and 14N is given by:
∆m = mass(14C) - mass(14N)
= 14.003241u - 14.003074u
= 0.000167u
The β-energy (Eβ) is related to the mass difference (∆m) by Einstein's mass-energy equivalence equation: E = ∆mc^2, where c is the speed of light.
Eβ = ∆m * c^2
Now, we need to convert the mass difference (∆m) from unified atomic mass units (u) to kilograms (kg) before using it in the equation. The conversion factor is approximately 1.66053906660 × 10^−27 kg/u.
∆m_kg = ∆m * (1.66053906660 × 10^−27 kg/u)
Next, we'll calculate the β-energy by substituting the values into the equation:
Eβ = ∆m_kg * c^2
The speed of light (c) is approximately 2.998 × 10^8 m/s.
Eβ = ∆m_kg * (2.998 × 10^8 m/s)^2
Let's perform the calculations:
∆m_kg = 0.000167u * (1.66053906660 × 10^−27 kg/u)
≈ 2.77273363362 × 10^−29 kg
Eβ = (2.77273363362 × 10^−29 kg) * (2.998 × 10^8 m/s)^2
≈ 2.49344368869 × 10^−13 J
The maximum β-energy in the decay of 14C to 14N is approximately 2.493 × 10^−13 Joules.
To know more about β - energy visit:
https://brainly.com/question/33544103
#SPJ11
The diagrams show gases that are stored in two separate but similar containers. 2 identical containers have gas particles, represented by small balls with arrows representing movement in random directions. The Gas 1 container has many fewer balls than the Gas 2 container. If both gases are at the same temperature, which one has the greater pressure? gas 1 because the particles are moving much faster gas 1 because it has fewer particles that are close together gas 2 because it has more particles that are colliding gas 2 because the particles have more space between them Mark this and return
Answers
Answer:
que es un compuesto ionico
When is a solution said to be supersaturated?
Answers
Explanation:
A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature.
1)
Layers of cells that form a basic structural material of a plant or animal are known as
A.) system
B.) organ
C.) tissue
D.) organism
Save
Not Graded
2)
All of these plant structures are used in reproduction except
A.) spore
B.) stamen
C.) pistil
D.) phloem
Save
Not Graded
3)
A nonliving or chemical factor that affects the ability of organisms to survive and reproduce is called
A.) a limiting factor
B.) a biotic factor
C.) an abiotic factor
D.) succession
Save
Not Graded
4)
The phyla that is the largest and most diverse is the
A.) sponges
B.) flatworms
C.) arthropods
D.) chordates
Save
Not Graded
5)
In order to survive all organisms must be able to do all of these except
A.) grow
B.) reproduce
C.) move from place to place
D.) eliminate wastes
Answers
Answer:
c
Explanation:
PLS HELP ASAP DUE AT 11:59 AND IM MARKING BRAINLIEST
PLS DO NOT ANSWER IF YOUR NOT SURE ABOUT YOUR ANSWER. this is science btw.

Answers
Answer:
In the followings below, the one that doesn't attract to magnets is:
Option 4, Aluminum.
Explanation:
Mainly because of the aluminum's crystal structure.
Hope this helped you out! ^^
#CarryOnLearning
Answer:
Aluminum
Explanation:
Ferromagnetic Metals
Ferromagnetic metals and alloys are the only magnetic metals. Out of all of the elements on the periodic table, there are only 3 metals that are attracted to magnets: iron, nickel, cobalt.
Aluminum
Aluminum is a nonferrous metal (meaning not including iron or steel) that does not include any ferromagnetic metals. Additionally, aluminum itself is not magnetic, so there is no attraction between aluminum and a magnet.
Which compound will contain the most polar bond? A. bromoethane, H3CCH2Br B. ethene, H2CCH2 C. ethane, H3CCH3 D. chloroethane, H3CCH2Cl
Answers
The compound that will contain the most polar bond is D. chloroethane, H3CCH2Cl. This is because the bond between the carbon and chlorine atoms is more polar than the bonds between the carbon and hydrogen atoms in the other compounds. The polarity of a bond is determined by the difference in electronegativity between the atoms involved in the bond. Chlorine has a higher electronegativity than carbon, so the bond between them will be more polar. The other compounds listed have bonds between carbon and hydrogen atoms, which have a smaller difference in electronegativity and are therefore less polar.
it is the minimum amount of solute that can be dissolved in the solvent
A. Salt Solution C. Saturated Solution
B. Sugar Solution D.
Answers
Answer:A
Explanation:
How many resonance structures can be drawn for co2−3?
Resonance:
Resonance is also called mesomerism. A compound shows resonance when it can be represented in more than one structure. The actual structure is a combination of all the structures.
Answers
The carbonate ion (CO₂³⁻) can be represented by three resonance structures.
Carbonate is a carbon oxoanion. It is a conjugate base of a hydrogencarbonate. ChEBI. Carbonate Ion is a polyatomic ion with formula of CO₃(²⁻).
Here are the three resonance structures for CO₂³⁻:
Structure with double bonds between carbon and two oxygen atoms:
O-C-O
Structure with double bonds between carbon and one oxygen atom, and a single bond with another oxygen atom:
O-C-O
Structure with double bonds between carbon and two oxygen atoms:
O-C-O
In reality, the actual structure of the carbonate ion is a combination or hybrid of these resonance structures, with the negative charge being delocalized over the three oxygen atoms. The image is given below.
To know more about double bonds:
https://brainly.com/question/14466514
#SPJ4

in general, which characteristics are necessary for a location to be suitable for chemical storage? select one or more: warm dark dry poorly-ventilated cool
Answers
Warm, dark, and poorly-ventilated locations are generally not suitable for chemical storage. it can be stored in cool places most of the time.
In general, a location suitable for chemical storage should be:
Cool: Many chemicals are temperature-sensitive and can degrade or react with other substances if exposed to high temperatures.
Dry: Moisture can cause some chemicals to react or degrade, and can also increase the risk of fire or explosion if electrical equipment is present.
Well-ventilated: Proper ventilation is important to ensure that fumes or vapors generated by the chemicals are safely dispersed.
Away from sources of heat and ignition: Chemicals should be stored away from sources of heat, sparks, and flames, to minimize the risk of fire or explosion.
Learn more about chemical storage here: brainly.com/question/8038400
#SPJ4
Complete question:
in general, which characteristics are necessary for a location to be suitable for chemical storage? select one or more:
A. Warm
B. Dark
C. Dry
D. Poorly-ventilated cool
Type the correct answer in the box. Isopropanol (C3H8O) is a key ingredient in some hand sanitizers. Suppose that 127 grams of isopropanol is dissolved in water. The volume of the solution is 1,250 milliliters. What is the molarity of the solution? Refer to the periodic table to help you answer. Express your answer to three significant figures. The molarity of the solution is M.
Answers
The molarity of the Isopropanol solution is 1.68 M.
How we calculate molarity?Molarity of any solution can be calculated as:
M = n/V, where
n = no. of moles
V = volume = 1,250mL = 1.250L
For this first we have to calculate the moles of Isopropanol (C₃H₈O), and it can be calculated as:
n = W/M, where
W = mass of C₃H₈O = 127 grams
M = molar mass of C₃H₈O = 60.1 g/mol
Moles of C₃H₈O = 127g / 60.1 g/mol = 2.1 moles
Now, we calculate the molarity of solution by using the above formula and by putting values as:
M = 2.1/1.250 = 1.68 M
Hence, 1.68 M is the molarity of the solution.
To know more about molarity, visit the below link:
https://brainly.com/question/24305514
In a 3.21g sample of the hydrate, CuSO4 • 10H2O (339.8 g/mol), how many grams of water are expected?
Answers
Therefore, 9.49 grams of water is expected in the given 3.21 g sample of CuSO4 • 10H2O.
To determine the number of water molecules in the given hydrate, CuSO4 • 10H2O, we'll need to find out the molar mass of the compound and the molar mass of water to make a comparison.
The molar mass of CuSO4 • 10H2O is calculated as:
CuSO4 → 159.6 g/mol10H2O → 180.16 g/mol (18.016 g/mol × 10)CuSO4 • 10H2O → 159.6 g/mol + 180.16 g/mol
= 339.76 g/mol (rounded to three significant figures)
Thus, we can see that the molar mass of CuSO4 • 10H2O is 339.76 g/mol.
We know that this hydrate consists of ten molecules of water, each having a molar mass of 18.016 g/mol (which is the same as the molar mass of water), and one molecule of CuSO4 with a molar mass of 159.6 g/mol.
Therefore, the number of moles of water in the sample is:
(10 × 18.016 g/mol) ÷ 339.76 g/mol = 0.527 moles
So, the mass of water is equal to its molar mass multiplied by the number of moles.
The mass of water is:
0.527 mol × 18.016 g/mol = 9.49 g
to know more about molecular mass visit:
https://brainly.com/question/15880821
#SPJ11
The equation for this reaction is MgO + H2O = MgO + h2. the teacher used 1.00g of magnesium. Calculate the maximum mass of MgO produced
Answers
The maximum mass of MgO produced is 1.64 g.
The given equation is not balanced. Assuming the correct equation is:
Mg + H₂O → MgO + H₂
The balanced equation shows that 1 mole of Mg reacts with 1 mole of H₂O to produce 1 mole of MgO and 1 mole of H₂.
The molar mass of Mg is 24.31 g/mol, which means that 1.00 g of Mg is equal to 1.00 g / 24.31 g/mol = 0.0411 mol Mg.
According to the balanced equation, 1 mol of Mg produces 1 mol of MgO. Therefore, 0.0411 mol of Mg produces 0.0411 mol of MgO.
The molar mass of magnesium oxide is 40.31 g/mol, which means that 0.0411 mol of MgO is equal to 0.0411 mol × 40.31 g/mol
= 1.64 g of MgO.
To know more about magnesium oxide here
https://brainly.com/question/1533548
#SPJ4
1. The pK, of acetic acid is 4.74. What is the buffering range of this acid and its conjugate base? Explain your answer. 2. For this experiment, you will need to prepare 100.00 mL of a 0.10 M CH3COOH solution. Calculate the volume of 6 M acetic acid needed to prepare this solution. 4. Determine the volumes of 0.10 M CH2COOH and 0.10 M CH3COONa required to prepare 10.0 mL of buffer of each of the following pH values. PK, of CH3COOH = 4.74 a. pH 3.7 b. pH 4.7 C. pH 5.7
Answers
The pKa of acetic acid is 4.74, 6 M acetic acid to prepare 100 mL of 0.10 M \(CH3COOH\) and 9.09 mL of 0.10 M \(CH_2COOH\) and 0.91 mL of 0.10 M CH3COONa.
1. The pKa of acetic acid is 4.74, which means its buffering range is between pH 4.74 and pH 5.74. A buffer is a solution that resists changes in pH when small amounts of acid or base are added. Acetic acid and its conjugate base, acetate, combine in solution to form a buffer. The amount of acetic acid and acetate will remain relatively constant over this pH range.
2. To prepare a 0.10 M \(CH3COOH\) solution, you need to calculate the volume of 6 M acetic acid needed.
Using the formula: \(M1V1 = M2V2\), you can calculate that you need 0.17 mL of 6 M acetic acid to prepare 100 mL of 0.10 M \(CH3COOH\).
3. To prepare 10.0 mL of buffer at the following pH values, you need to calculate the volume of 0.10 M\(CH_2COOH\) and 0.10 M \(CH3COONa\). At pH 3.7, you will need 4.74 mL of 0.10 M CH2COOH and 5.26 mL of 0.10 M CH3COONa. At pH 4.7, you will need 6.45 mL of 0.10 M CH2COOH and 3.55 mL of 0.10 M CH3COONa. At pH 5.7, you will need 9.09 mL of 0.10 M CH2COOH and 0.91 mL of 0.10 M CH3COONa.
To know more about buffer here:
brainly.com/question/22821585#
#SPJ11
1. The pKa of acetic acid is 4.74, which means its buffering range is between pH 4.74 and pH 5.74. A buffer is a solution that resists changes in pH when small amounts of acid or base are added. Acetic acid and its conjugate base, acetate, combine in solution to form a buffer. The amount of acetic acid and acetate will remain relatively constant over this pH range.
2. To prepare a 0.10 M CH3COOH solution, you need to calculate the volume of 6 M acetic acid needed. Using the formula: M1V1 = M2V2, you can calculate that you need 0.17 mL of 6 M acetic acid to prepare 100 mL of 0.10 M CH3COOH.
3. To prepare 10.0 mL of buffer at the following pH values, you need to calculate the volume of 0.10 M CH2COOH and 0.10 M CH3COONa. At pH 3.7, you will need 4.74 mL of 0.10 M CH2COOH and 5.26 mL of 0.10 M CH3COONa. At pH 4.7, you will need 6.45 mL of 0.10 M CH2COOH and 3.55 mL of 0.10 M CH3COONa. At pH 5.7, you will need 9.09 mL of 0.10 M CH2COOH and 0.91 mL of 0.10 M CH3COONa.
To know more about buffer click here:
https://brainly.com/question/22821585
#SPJ11
the element to atomic 35 belong to_____ A, s-block B,P-block c, d-block D,f-block
Answers
Answer:
B
Explanation:
p block bc its last orbit is 4p5
Answer:
Explanation:
P. block
50 POINTS pls answer the full thing<333 i will report if you don't (will mark barinliest)
Some greenhouse gases, such as fluorocarbons (CFCs, HFCs, PFCs, etc.), are human-made. Others, such as water, methane, and carbon dioxide, are naturally produced. Which type of greenhouse gas (human-made or natural) is more difficult to control and eliminate? Which types are easier? In three to five sentences, provide evidence for your argument.(4 points)
Wetlands are able to remove nutrients and chemicals from water as the water flows through the area. A developer is planning to destroy most of the wetlands near a bay. In three to five sentences, explain how destroying the wetlands would impact the bay’s water quality and ecosystem.(4 points)
Commercial agriculture can often lead to water-quality problems. In one to two sentences, explain how two of those problems occur.(2 points)
Answers
Destroying the wetlands would result in a significant decline in the bay's water quality and ecosystem. Wetlands are natural filters that remove nutrients and chemicals from water as it flows through the area. By destroying the wetlands, the water quality of the bay would decline as pollutants and chemicals would no longer be filtered out. This would have a significant impact on the bay's ecosystem, as many species rely on the bay's water quality to survive.
Two water-quality problems that can arise from commercial agriculture are eutrophication and contamination from pesticides and fertilizers. Eutrophication is the process by which excess nutrients enter a body of water, leading to the growth of algae and other aquatic plants. This can lead to a depletion of oxygen in the water, which can harm aquatic life. Pesticides and fertilizers used in commercial agriculture can also contaminate water sources, leading to health problems for humans and animals that rely on the water.