Answers
Answer:
57.9 grams .
Explanation:
Ca₃(PO₄)₂ + 3H₂SO₄ → 3CaSO₄ + 2H₃PO₄
1 mole 3 moles 2 moles
149 g of Ca₃(PO₄)₂ = 149 / 310 = .48 moles
86.9 g of H₂SO₄ = 86.9 / 98 = .8867 moles
Here H₂SO₄ is the limiting reagent .
.8867 / 3 = .2955
.2955 moles of Ca₃(PO₄)₂ reacts with .8867 mole of H₂SO₄ to give
2 x .2955 moles of H₃PO₄
H₃PO₄ produced = 2 x .2955 moles
= .591 moles
= .591 x 98 = 57.9 grams .
Related Questions
the pressure on 20 milliliters of a gas at constant temperature is changed from 4 atmospheres to 2 atmospheres. what is the new volume of the gas?
Answers
The new volume of the gas whose pressure was changed would be = 40 milliliters.
How to calculate the new volume of the given gas?The initial volume(V1)of the gas= 20ml
The initial pressure(P1) = 4 atm
The final pressure(P2) = 2 atm
The final volume(V2) = ?
Using the general gas formula;
P1V1 = P2V2
V2 = P1V1/P2
= 4×20/2
= 40ml
Learn more about volume here:
https://brainly.com/question/27710307
#SPJ1
Gas Laws
Pre-Test Active
1
2 3
5
6
O final pressure
O atmospheric pressure
O combined pressure
O partial pressure
7 8
9
10
A scientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide,
oxygen, and nitrogen. Which term most likely describes what she is measuring?
Answers
The term that the scientist would use in this case is partial pressure. Option D
What is the partial pressure?
The pressure that one particular gas component within a mixture of gases exerts is referred to as partial pressure. It is the pressure that the gas would experience at the same temperature if it were the only thing in the entire volume.
When researching gas mixtures, such as in gas laws, gas phase equilibria, and gas collecting methods, partial pressures are extremely crucial for the gas.
Learn more about partial pressure:https://brainly.com/question/30114830
#SPJ1
Use the information in table to find standard enthalpy of the combustion of methane and also create a hess enthalpy diagram for reaction.
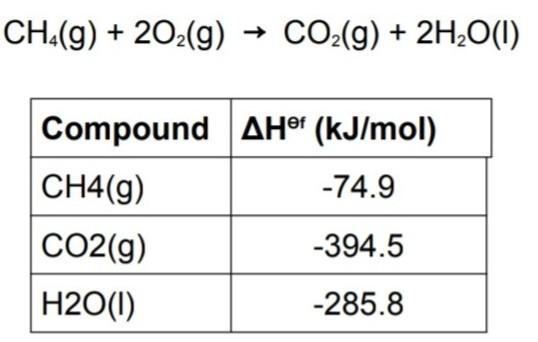
Answers
The combustion of methane can be represented by the equation CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)We are to find the standard enthalpy of combustion of methane.
We are also to create a Hess enthalpy diagram for the reaction. Standard enthalpy of combustion is the amount of energy released when one mole of a substance is completely burned in excess oxygen at standard temperature and pressure. We can use the data in the table to calculate the standard enthalpy of combustion of methane.The table gives us the standard enthalpies of formation of the reactants and products. The standard enthalpy of formation is the amount of energy change that occurs when one mole of a compound is formed from its constituent elements in their standard states (usually at 25°C and 1 atm).We can use the standard enthalpies of formation to calculate the standard enthalpy of combustion of methane. The standard enthalpy of combustion is given by the following equation:ΔH°c = ΣΔH°f(products) – ΣΔH°f(reactants)We can calculate the standard enthalpy of combustion of methane using the standard enthalpies of formation from the table as follows:ΔH°c = [ΔH°f(CO2(g)) + 2ΔH°f(H2O(g))] – [ΔH°f(CH4(g)) + 2ΔH°f(O2(g))]Substituting the values from the table:ΔH°c = [(–393.5 kJ/mol) + 2(–241.8 kJ/mol)] – [(–74.8 kJ/mol) + 2(0 kJ/mol)]ΔH°c = (–890.2 kJ/mol) – (–74.8 kJ/mol)ΔH°c = –815.4 kJ/mol. Therefore, the standard enthalpy of combustion of methane is –815.4 kJ/mol.We can create a Hess enthalpy diagram for the reaction as shown below: The Hess enthalpy diagram is used to show how the standard enthalpy of the reaction can be calculated using the standard enthalpies of formation of the reactants and products. The diagram shows the two steps involved in the reaction. The first step involves the breaking of the bonds in methane and oxygen to form the reactants. The second step involves the formation of the products from the reactants. The standard enthalpy of combustion is the difference between the standard enthalpies of formation of the products and reactants. The Hess enthalpy diagram shows that the same standard enthalpy of combustion can be obtained regardless of the pathway taken to get from reactants to products.
for such more questions on equation
https://brainly.com/question/26694427
#SPJ8
The boiling point of another member of this homologous series was found to be 309 KK. What is the likely molecular formula for this compound?
Answers
Answer: Pentane C5H12
Explanation:
The boiling point of a substance is simply defined as the temperature whereby a liquid's vapor pressure is equal to the pressure that is surrounding the liquid and hence, the liquid will changes into vapor.
The likely molecular formula for this compound is Pentane i.e C5H12 due to the fact that its boiling point is between Butane with formula C4H10 and Hexane with formula C6H14 boiling points.
The molecular formula of the of the substance is \(\bold {C_5H_1_0}\) (pentane).
Boiling point:
It is the temperature at which liquid's vapor pressure is equal to the pressure that is surrounding the liquid and hence, the liquid start to into vaporize.
The boiling point of the given compound is 309 K which is between Butane and Hexane.
Therefore, the molecular formula of the of the substance is \(\bold {C_5H_1_0}\) (pentane).
To know more about Boiling point,
https://brainly.com/question/2153588
A gas has a solubility of 2.45 g/L at a pressure of 0.750 atm. What pressure wold be required to produce an aqueous solution containing 6.25 g/L of this gas at constant temperature
Answers
Answer:
1.91 atm
Explanation:
Step 1: Calculate Henry's constant (k)
A gas has a solubility (C) of 2.45 g/L at a pressure (P) of 0.750 atm. These two variables are related to each other through Henry's law.
C = k × P
K = C/P
K = (2.45 g/L)/0.750 atm = 3.27 g/L.atm
Step 2: Calculate the pressure required to produce an aqueous solution containing 6.25 g/L of this gas at constant temperature.
We have C = 6.25 g/L and k = 3.27 g/L.atm. The required pressure is:
C = k × P
P = C/k
P = (6.25 g/L)/(3.27 g/L.atm) = 1.91 atm
what is the the correct equation for water gas
Answers
Answer:
Water-gas A mixture of carbon monoxide (CO) and hydrogen (H2) produced by passing steam over red-hot coke using the endothermic reaction C + H2O → CO + H2.
What does a compound do in a chemical reaction?
Answers
Answer:Compounds are chemical substances that contain more than one element. They're created during a chemical reaction where atoms are rearranged into new compound molecules. For example, if carbon atoms react with oxygen atoms they form carbon dioxide molecules.
Explanation:
Please help me, I’m literally so lost!!

Answers
2. 30g divided by 48.06 ≈ 0.62 mol
[H+] for a solution is2.65 x 10-2 M.This solution is
![[H+] for a solution is2.65 x 10-2 M.This solution is](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/HF4dTPPHgHDRHmVemF5Z4aeiUX4EmO36.jpeg)
Answers
Answer
A. Acidic
Explanation
Given:
[H⁺] = 2.65 x 10⁻² M
Note that: the pH values range from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base.
Also, the pH of a solution can be determine using the pH formula below:
\(pH=-\log \lbrack H^+\rbrack\)So pH = - log [2.65 x 10⁻²]
pH = 1.58
The pH of the solution fall in the acidic range. Hence, the solution acidic.
In laboratory experiment, a NOVDEC Student was
required to prepare 500 cm3 of Im Solution of
glucose (c6, H12,06) Determine the
i Molar
Mass
ii) Amount of ghicoseB. In in moles in the Solrition
[ C= 12, H = 10, 0=16]
Answers
Answer:
i. Molar mass of glucose = 180 g/mol
ii. Amount of glucose = 0.5 mole
Explanation:
The volume of the glucose solution to be prepared = 500 \(cm^3\)
Molarity of the glucose solution to be prepared = 1 M
i. Molar mass of glucose (\(C_1_2H_6O_6\)) = (6 × 12) + (12 × 1) + (6 × 16) = 180 g/mol
ii. mole = molarity x volume. Hence;
amount (in moles) of the glucose solution to be prepared
= 1 x 500/1000 = 0.5 mole
Help please!!!!!!!!!!!

Answers
water acts as the solvent for this experiment
we use to calculate R f values and compare with know to determine the compound is made of example in forensic science to find DNA, amino acids
most soluble one will travel the most
c to b to a (A most further travelled)
Explanation:
This quest in requires general knowledge on chromatography
For the reaction shown, identify the oxidation half-reaction and the reduction half-reaction. KNO3 → KNO2 + O₂ Which pair of half-reactions represents the balanced half-reactions? N5+ + 2e →N³+ 0² →0₂ + 4e 2N5+ + 4e¯ → 2N³+ 20²- → 0₂ +4e 4N5+ 2e4N³+ 20² → 20₂ + 4e¯
Answers
N5+ + 2e →N³
here Nitrogen gains electron so, its reduction half-reaction
20²- → 0₂
here oxygen loss electron so, its oxidation half-reaction
20² → 20₂ + 4e¯
here oxygen loss electron so, its oxidation half-reaction
2N5+ + 4e¯ → 2N³
here nitrogen gains electron so, its reduction half-reaction
When an atom loses electrons, it is said to be oxidising; when it acquires electrons, it is said to be reduced. A redox reaction occurs when both of these reactions take place at the same time.When a molecule oxidises, it implies it either gives or loses electrons to another molecule. As a result, the reaction between a molecule of oxygen and a molecule of hydrochloric acid may be shown below. Because it has to combine with hydrogen to create a proper molecule, the oxygen molecule in this process is unstable. Four extra hydrogen molecules are available for disposal in the hydrogen chloride molecule. Thus, although the hydrochloric acid transforms into chlorine gas, all of the hydrogen from the acid unites with the oxygen molecule to form water.To know more about oxidation visit : https://brainly.com/question/13003361
#SPJ9
which of the following is an example of
a physical property?
Answers
Answer:
physical property is property that you can touch, smell as it is bumpy it smells like eggs.
Explanation:
YW
Answer:
Physical properties include: appearance, texture, color, odor, melting point, boiling point, density, solubility, polarity, and many others.
Explanation:
How many atoms of Hydrogen are in C₆H₁₂O₆?
Answers
Question 3 of 10
What Group is F (fluorine) in?
O A. ZB
O B. 2
O C. 17
O D. 9
Answers
Answer:
C
Explanation:
Answer:
ganyan din ang alam ko
Explanation:
letter c
How many grams of NaCl

Answers
You would recover 36.525g of NaCl after evaporating all of the water.
How to find the how many grams of NaCl that would be recover when all water is evaporated off of this solution?To find the grams of NaCl that would be recovered after evaporating all the water, we can use the following formula:
mass = moles * molar mass
Where:
Moles = Molarity * Volume
Molarity = 0.250 M
Volume = 2500.0 mL = 2.5 L
Molar mass of NaCl = 58.44 g/mol
mass = 0.250 M * 2.5 L * 58.44 g/mol
mass = 36.525 g
Learn about evaporation here https://brainly.com/question/2013258
#SPJ1
for a molecule to be studied in IR spectra?
Answers
The molecule must possess dipolar bonds, exhibit suitable vibrational frequencies, and be in a form that can be analyzed using IR spectroscopy in order to be studied in an IR spectrum.
To study a molecule in an infrared (IR) spectrum, several criteria need to be considered. Firstly, the molecule should have a dipole moment, meaning it must have a separation of positive and negative charges within the molecule.
This is because IR spectroscopy primarily detects vibrations of covalent bonds, which are associated with changes in dipole moment.Additionally, the molecule should have covalent bonds that can undergo vibrational modes within the range of the instrument. IR spectroscopy typically covers the frequency range of 4000 to 400 cm⁻¹, which corresponds to the energies of molecular vibrations.
The molecule should also be able to be vaporized or dissolved in a suitable solvent to generate a homogeneous sample for analysis. Gaseous or liquid samples are commonly used in IR spectroscopy.
For more such questions on dipolar bonds
https://brainly.com/question/20432466
#SPJ11
A 0.457-M aqueous solution of (CH3)2NH (dimethylamine) has a pH of 12.2. Calculate the pH of a buffer solution that is 0.457 M in (CH3)2NH and 0.280 M in (CH3)2NH2 .
Answers
Answer:
pH = 10.95
Explanation:
To solve this question we must, as first, find pKb of dimethylamine. Then, using H-H equation we can solve the pH of the buffer:
pKb dimethylamine:
Based on the equilibrium:
(CH3)2NH(aq) + H2O(l) ⇄ (CH3)2NH2⁺(aq) + OH-(aq)
Kb is defined as:
Kb = [OH-] [(CH3)2NH2⁺] / [(CH3)2NH]
Both (CH3)2NH2⁺(aq) + OH- comes from the same equilibrium, that means:
[OH-] = [(CH3)2NH2⁺]
And: [(CH3)2NH] = 0.457M
[OH-] can be obtained from pH as follows:
14 -pH = pOH
14-12.2 = 1.8 =pOH
10^-pOH = [OH-] = 0.01585M
Replacing:
Kb = [0.01585M] [(0.01585M] / [0.457M]
Kb = 5.50x10⁻⁴
pKb = -logkb = 3.26
pH of the buffer:
Using H-H equation for bases:
pOH = pKb + log [conjugate acid] / [weak base]
pOH = 3.26 + log [0.280M] / [0.457M]
pOH = 3.05
pH = 14 - pOH
pH = 10.95How much heat is required to melt 38.6 g of ice (0 degrees Celsius)? The heat of fusion for water is 6.02 kJ/mol.
Express your answer in kilojoules to three significant figures
Answers
Given the heat of fusion of water is 6.02 kJ/mol, it takes 12.9 kJ of heat to melt 38.6 g of ice at 0 °C.
What is fusion?Fusion, commonly known as melting, is a physical process in which matter passes from the solid to the liquid state.
We have ice with a mass of 38.6 g at 0 °C. First, we will convert mass to moles using the molar mass of water.
38.6 g × (1 mol/18.02 g) = 2.14 mol
The heat of fusion (ΔH°fus) for water is 6.02 kJ/mol. The heat (Q) required to melt 2.14 moles of ice is:
Q = 6.02 kJ/mol × 2.14 mol = 12.9 kJ
Given the heat of fusion of water is 6.02 kJ/mol, it takes 12.9 kJ of heat to melt 38.6 g of ice at 0 °C.
Learn more about fusion here: https://brainly.com/question/40140
#SPJ1
A softball is thrown with 145 Joules of kinetic energy. If the ball is moving at 20.0 m/s, what is the mass of the ball in kg?
Answers
Answer:
0.725 kg
Explanation:
Step 1: Given data
Kinetic energy of the softball (K): 145 JSpeed of the softball (v): 20.0 m/sMass of the softball (m): ?Step 2: Calculate the mass of the softball
We will use the following expression.
K = 1/2 × m × v²
m = 2 K / v²
m = 2 × 145 J / (20.0 m/s)²
m = 0.725 kg
The mass of the softball is 0.725 kg.
Are any of these equations redox reactions? if so what are their reducing and oxidizing agents?
7O2(g)+2CH3CH3(g)→4CO2(g)+6H2O(g)
HSO-4(aq)+OH-(aq)→SO42-(aq)+H2O(l)
Fe(s)+CuSO4→FeSO4(aq)+Cu(s)
Answers
Yes, Fe(s)+CuSO4→FeSO4(aq)+Cu(s) is a redox reaction. The reducing agent is Fe(s), while the oxidizing agent is CuSO4.
Oxidation-reduction reaction (redox)Oxidation-reduction reaction is the type of reaction in which electrons are transferred from one element to another. A compound or an element either gains are looses an electron in redox reactions.
Fe(s)+CuSO4→FeSO4(aq)+Cu(s)
From the equation above, Fe(s) is the reducing agent because it gives away 2 electrons and undergoes oxidation reaction.
From the equation above, CuSO4 is an oxidizing agent because it gives oxygen to Fe(s) and undergoes a reduction reaction.
Therefore, the reducing agent is Fe(s), while the oxidizing agent is CuSO4.
Learn more about redox reactions here:
https://brainly.com/question/26263317
Science pls helppp?!

Answers
Answer:
2nd one down
Explanation: distance divided by time interval
Which bone is located between the incus and the inner ear?
cochlea
stapes
incus
malleus
Answers
Answer: The answer is incus
what is the ratio of atoms of potassium to atoms of oxygen
Answers
Ratio of number of atoms of K,Cl & O=1:1:3 .
Which of the following molecules are considered inorganic?
A.)DNA
B.)Lipids
C.)Proteins
D.)Water
Answers
Answer:
the answer is D . water
Explanation:
the others are organic molecules

Electrochemical cells generate electricity from which of the following? Select all that apply.
electron transfer
flow of electrons
dissolving an ionic compound
redox reactions
Answers
By a redox reaction that involves the transfer of electrons, often through the dissolution of an ionic substance, electrochemical cells produce electricity from the flow of electrons.
What fuels the production of energy by electrochemical cells?In electrochemistry, redox or oxidation-reduction reactions, in which electrons travel from one element to another, can produce electricity. Redox processes involve the transfer of electrons from one substance to another.
In what element are electrochemical cells made?Batteries use a very significant class of oxidation and reduction reactions to produce useable electrical energy. Using solutions of respective sulphates, copper and zinc metals can be combined to create a straightforward electrochemical cell.
To know more about electrons visit:-
https://brainly.com/question/20513633
#SPJ1
consider a solution containing alcohol and water. If the mole fraction of water is 0.600, what is the mole fraction of alcohol?
Answers
Consider a solution containing alcohol and water. If the mole fraction of water is 0.600, 0.4 is the mole fraction of alcohol.
What is mole fraction?A mole fraction is indeed a measurement of concentration that is equal to the product of the moles of such a component and the total moles of the solution.
Mole fraction is indeed a unitless phrase since it represents a ratio. When all the parts of a solution's mole fraction are summed up, they equal one.
mole fraction of water + mole fraction of alcohol =1
mole fraction of water=0.600
mole fraction of alcohol =1
mole fraction of alcohol =1-0.600 =0.4
Therefore, 0.4 is the mole fraction of alcohol.
To know more about mole fraction, here:
https://brainly.com/question/29808190
#SPJ1
Which of the following is an incorrect representation for a neutral atom?
36Li
613C
3063Cu
1530P
Answers
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97. The incorrect representation for a neutral atom is 36Li
To determine the correct representation for a neutral atom, we need to consider the atomic number (Z) and mass number (A) of the element. The atomic number represents the number of protons in the nucleus, while the mass number represents the sum of protons and neutrons.
Let's analyze the given representations:
36Li:
This representation suggests that the element is lithium (Li) with a mass number of 36, which is incorrect. The correct mass number for lithium is approximately 6.94.
613C:
This representation suggests that the element is carbon (C) with a mass number of 13, which is correct. Carbon has different isotopes, and 13C represents one of its stable isotopes.
3063Cu:
This representation suggests that the element is copper (Cu) with a mass number of 63, which is correct. Copper has different isotopes, and 63Cu represents one of its stable isotopes.
1530P:
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97.
Therefore, the incorrect representation for a neutral atom is 36Li, as it does not match the known properties of lithium.
For more question on atom
https://brainly.com/question/26952570
#SPJ8
What is the standard enthalpy change for the reaction:
3NO2(g)+H2O(l)→2HNO3(aq)+NO(g)
Use standard enthalpies of formation in your textbook.
Select one:
a.
-66.2 kJ
b.
-138 kJ
c.
-116 kJ
d.
-201 kJ
Answers
Question 1
Given the equation: Q = mcAT
Q = heat (in Joules)
m = mass (in grams)
C = 4.18 (specific heat capacity)
AT change in temperature (°C)
How many Joules of heat energy are absorbed when 200 grams of water are heated from 20 C to 60 C.

Answers
The amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C is 33,440 Joules.
To find the amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C, we can use the equation Q = mcAT.
First, we need to find the value of m, which is the mass of the water in grams. In this case, it is given as 200 grams.
Next, we need to find the value of AT, which is the change in temperature in degrees Celsius.
This can be calculated by subtracting the initial temperature from the final temperature, which gives us 60 C - 20 C = 40 C.
The specific heat capacity of water, C, is given as 4.18 Joules per gram per degree Celsius.
Now we can plug in the values into the equation:
Q = mcAT
Q = (200 g) x (4.18 J/g°C) x (40°C)
Q = 33,440 J
Therefore, the amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C is 33,440 Joules.
for more such question on heat energy
https://brainly.com/question/25603269
#SPJ8