Substances A₂, B₂, and C₂ can all act as oxidizing agents. In solution, A₂ is green, B₂ is yellow, and C₂ is red. In the reactions in which they participate, they are reduced to A,B, and C ions, all of which are colorless. When a solution of B2 is mixed with one containing C to red. ions, the color changes from yellow a. Which species is oxidized? Which is reduced? When a solution of B2 is mixed with one containing A ions, the color remains yellow. Which species is a better oxidizing agent, B₂ or A₂ ?
Which species is a better oxidizing agent B₂ or C₂?
Arrange A₂, B₂, and C₂ in order of increasing strength of oxidizing agent. weakest strongest
Answers
The order of increasing strength of oxidizing agent is A₂ < C₂ < B₂, with A₂ being the weakest and B₂ being the strongest. In the reaction between B₂ and C ions, B₂ is being oxidized because it is losing electrons and causing the color change from yellow to red. C ions are being reduced because they are gaining electrons.
In the reaction between B₂ and A ions, the color remains yellow, indicating that no oxidation or reduction is taking place. Therefore, neither B₂ nor A₂ is a better oxidizing agent than the other.
C₂ is a better oxidizing agent than B₂ because it is able to cause a color change in the presence of B₂, while B₂ cannot cause a color change in the presence of A₂.
The order of increasing strength of oxidizing agent is A₂, B₂, C₂, with A₂ being the weakest and C₂ being the strongest.
In the first reaction, when a solution of B₂ (yellow) is mixed with C ions (colorless), the color changes to red, which is the color of C₂. This means that B₂ is reduced to colorless B ions, and C ions are oxidized to C₂. Therefore, B₂ is the oxidizing agent and C is the reducing agent in this reaction.
In the second reaction, when a solution of B₂ (yellow) is mixed with A ions (colorless), the color remains yellow, which indicates that no reaction occurs. This suggests that A₂ is a weaker oxidizing agent than B₂ since it cannot oxidize B ions.
To compare B₂ and C₂ as oxidizing agents, we can see that B₂ is able to oxidize C ions, while A₂ cannot oxidize B ions. Therefore, B₂ is a stronger oxidizing agent than A₂. Since A₂ is weaker than B₂, and B₂ can oxidize C ions, we can infer that B₂ is also a stronger oxidizing agent than C₂.
So, the order of increasing strength of oxidizing agent is A₂ < C₂ < B₂, with A₂ being the weakest and B₂ being the strongest.
For more information on oxidizing agent visit:
brainly.com/question/10547418
#SPJ11
Related Questions
Differentiate the 3 types of evolution in Science
Answers
Answer:
Brainliest pls
Explanation:
shows the three main types of evolution: divergent, convergent, and parallel evolution.
Divergent: tending to be different or develop in different directions.
Convergent: coming closer together, especially in characteristics or ideas.
Parallel evolution: implies that two or more lineages have changed in similar ways
Rank lead, phosphorus, and barium in order of increasing ionization energy.
Answers
Answer:
D) Ba < Pb < P
Explanation:
Barium (Ba): 5,2117
Lead (Pb): 7,4167
Phosphorus (P): 10,4867
The amino acid glycine can be condensed to form a polymer called polyglycine. Draw the repeating monomer unit
Answers
The repeating monomer unit of polyglycine is simply the amino acid glycine. The chemical structure of glycine is:
H
|
H2N — C — COOH
|
H
The polymerization of glycine involves the condensation of the amino group (-NH2) of one glycine molecule with the carboxyl group (-COOH) of another glycine molecule, releasing a molecule of water (H2O) in the process. The resulting bond is called a peptide bond, and it connects the carbon atom of one glycine molecule to the nitrogen atom of the other glycine molecule.
The repeating monomer unit of polyglycine can be represented as:
H H H
| | |
H2N — C — CO — NH — C — CO — NH — C — COOH
| |
H H
Note that the NH group on the left side of the monomer unit represents the amino group of one glycine molecule, and the CO group on the right side represents the carboxyl group of the other glycine molecule. This pattern repeats indefinitely to form the polyglycine polymer.
#SPJ1
PLEASE HELP CHEMISTRY

Answers
Answer:
charge of copper.. ........mark me brainlest
Return all unused chemicals to their original
containers.
1. T
2. F
Answers
Answer: F
Explanation:
Predict whether or not the substances in the table will sublime at STP. Base your predictions only on the type of force holding the solid together.

Answers
The states of matter of the materials;
1) Dispersion forces - Yes
2) Hydrogen bonding - No
3) Ionic - No
4) Dispersion forces - No
5) Dispersion forces - Yes
6) Ionic - No
7) Hydrogen bonding - No
What is the sublimation?
Sublimation is a physical process in which a substance transitions directly from its solid phase to its gaseous phase without passing through the intermediate liquid phase. In sublimation, the solid substance is heated, and the resulting gas molecules escape from the solid lattice structure without the need for melting.
One common example of sublimation is the process of dry ice. Dry ice is solid carbon dioxide (CO2) that sublimes at a temperature of -78.5 degrees Celsius (-109.3 degrees Fahrenheit). When dry ice is exposed to room temperature, it transitions directly from a solid to a gas, producing a fog-like effect.
Learn more about states of matter:https://brainly.com/question/9402776
#SPJ1
Water 3.0 deals mainly with sewage treatment.
Describe which chemicals are currently not broken down by currently
used wastewater technologies and why that is important.
Answers
Water 3.0 deals mainly with sewage treatment. The primary aim of this project is to reduce the harmful impacts of chemical pollutants from industrial and agricultural activities on natural water resources.
Currently, used wastewater treatment technologies can break down some of the chemicals in wastewater but not all of them. Chemicals that are not broken down are referred to as persistent organic pollutants. These chemicals persist in the environment for long periods, and they can cause severe damage to aquatic life and human health.
Currently, the primary challenge facing water treatment technologies is the removal of persistent organic pollutants such as pesticides, pharmaceuticals, and endocrine-disrupting chemicals from wastewater.
These pollutants are generally water-soluble and resist microbial degradation, making them hard to remove from wastewater using current water treatment technologies. For example, conventional activated sludge treatment used in wastewater treatment plants does not remove some persistent organic pollutants from wastewater.
Failure to remove these pollutants from wastewater can have significant environmental and health impacts.
For example, pharmaceutical chemicals can cause antibiotic resistance, while endocrine-disrupting chemicals can cause birth defects, cancer, and other health problems.
Therefore, there is a need to improve wastewater treatment technologies to remove persistent organic pollutants from wastewater.
In conclusion, wastewater treatment technologies can break down some chemicals but not all. Chemicals that are not broken down are persistent organic pollutants and pose a significant risk to the environment and human health. Therefore, it is important to develop wastewater treatment technologies that can remove these pollutants from wastewater.
To know more about chemicals visit:
https://brainly.com/question/29240183
#SPJ11
Below is the structure for the amino acid glycine. Which bond angles are closest to the actual values for the h-n-c and o-c-o bond angles? consider all lone pairs of electrons as substituents when answering this question.
Answers
We have that the bond angles that are closest to the actual values for the h-n-c and o-c-o bond angles are
109.5 degrees.120 degrees.H-N-C and O-C-O bond anglesGenerally, In H-N-C bond nitrogen has Sp3 hybridization, it will have the angle 109.5 degrees.
In O-C-O bond C atom has sp^2 Hybridization so it will have the angle by 120 degrees.
Therefore,bond angles that are closest to the actual values for the h-n-c and o-c-o bond angles are
109.5 degrees.120 degrees.For more information on Bonding visit
https://brainly.com/question/819068
What is the [OH) if the poH is 4.9?
a) 4.9 x 10-10 M
Ob) 1.0 x 10-4 M
C) 1.25 x 10-5 M
O d) 7.94 x 10-10 M
Answers
Answer:
the answer is 4.9×10-10M
If oxygen has an atomic number of 8 and an atomic mass of 16, how many protons, neutrons and electrons would oxygen have?
Answers
Answer:
The oxygen we breathe is not in atomic form. It is in its molecular form, which is two oxygen atoms joined together by bonds, and between its two atoms, this molecule has 16 protons, 16 electrons, and most commonly 16 neutrons. Answer 5: An oxygen atom contains eight protons.
The strength of dipole interactions are mostly determined by what?
a. The difference in electronegativity between the two bonded atoms.
b. The difference in sizes between the two bonded atoms.
c. The difference in the phase the bonded molecule is in
Answers
The strength of dipole interactions are mostly determined by the difference in electronegativity between the two bonded atoms i.e. option A is correct.
Electronegetivity is the measure of the ability of the atoms to attract the electrons towards itself. If there exists electronegativity difference between the atoms in a covalant comound, the electrons will be unequally shared. It will lead to the formation of partial positive and negetive charges. This ultimately causes dipole movement. Dipole movement is the seperation of electrical charges of the atoms in a molecule.
Greater the electronegativity difference amongst the atoms of a comound, stronger will be the dipole-dipole interaction between the molecules.
Therefore, the strength of dipole movement are mostly determined by the difference in electronegativity between the two bonded atoms.
Learn more anout dipole movement :
https://brainly.com/question/30415832
#SPJ4
A 5.0-mL sample of CO2 gas is enclosed in a gas-tight
syringe (Figure 11.3) at 22 °C. If the syringe is
immersed in an ice bath (0 °C), what is the new gas
volume, assuming that the pressure is held constant?
Answers
Answer:
3.5ML
Explanation:
At the constant pressure, the final volume of the carbon dioxide gas was equal to 4.62 ml at a temperature of 0ºC.
What is Charles's law for volume-temperature relation?Charles's law can be explained as when the pressure of the gas is kept constant, the volume of gas is directly proportional to the temperature of a gas.
The volume of the gas and the temperature of the gas have a direct relationship according to this law.
Therefore, V∝ T
or V₁/T₁ = V₂/T₂ ....................(1)
Given, the initial temperature of the CO₂ gas, T₁ = 22 ºC
T₁ = 22 + 273 = 295 K
The final temperature of the CO₂ gas, T₂ = 0ºC
T₂ = 0 + 273 = 273 K
The initial volume of the gas, V₁ = 5 ml
Substitute the value of V₁, T₁, and T₂ in equation (1 ) to get final volume of CO₂ gas:
5/295 = V₂/273
V₂ = 4.6 ml
Therefore, the final volume of the gas is equal to 4.6 ml at 0 ºC, when there is no change in the pressure.
Learn more about Charles's law, here:
brainly.com/question/16927784
#SPJ2
nitrogen-13 has a half-life of 10 minutes. how much of a 200 mg sample would remain after 30 minutes?
Answers
The Nitrogen-13 has a half-life of 10 minutes0.250 g of a 200 mg sample would remain after 30 minutes .
Calculation :
Determine the remaining mass of the substance,
mf if the initial mass is mi = 2.00 g. After 30 minutes, 30 minutes/10 minutes = 3 half-lives have passed, so the residual mass is reduced by a factor of i/2³. Calculate the residual mass of a substance using the formula, mf=mi×1/2³. We proceed with the solution.
mf=mi×1/2³
mf=2.00g/8
=0.250g
Half-life (symbol t½) is the time it takes for a (substance) quantity to reduce its original value by half. The term is often used in nuclear physics to describe how quickly unstable atoms radioactively decay or how long stable atoms survive.
The term is also used more generally to characterize any type of exponential (or rarely non-exponential) decay. For example, medicine is concerned with the biological half-life of drugs and other chemicals in the human body. The opposite of half-life (when growing exponentially) is doubling time.
Learn more about half-life here : https://brainly.com/question/1160651
#SPJ4
calculate e.e. of a mixture containing 5.70 g of (-)-glyceraldehyde and 2.0 g of ( )-glyceraldehyde.
Answers
The enantiomeric excess (e.e.) of the mixture containing 5.70 g of (-)-glyceraldehyde and 2.0 g of (+)-glyceraldehyde is 48%.
To calculate the enantiomeric excess (e.e.) of a mixture, we need to determine the difference in the amounts of the two enantiomers and express it as a percentage of the total amount.
Given;
Mass of (-)-glyceraldehyde = 5.70 g
Mass of (+)-glyceraldehyde = 2.0 g
Calculate the total amount of glyceraldehyde in the mixture.
Total mass of glyceraldehyde = Mass of (-)-glyceraldehyde + Mass of (+)-glyceraldehyde
= 5.70 g + 2.0 g
= 7.70 g
Calculate the individual amounts of each enantiomer as a fraction of the total amount.
Fraction of (-)-glyceraldehyde = Mass of (-)-glyceraldehyde / Total mass of glyceraldehyde
= 5.70 g / 7.70 g
= 0.740
Fraction of (+)-glyceraldehyde = Mass of (+)-glyceraldehyde / Total mass of glyceraldehyde
= 2.0 g / 7.70 g
= 0.260
Calculate the enantiomeric excess (e.e.).
e.e. = (Fraction of (-)-glyceraldehyde - Fraction of (+)-glyceraldehyde) × 100%
= (0.740 - 0.260) × 100%
= 0.480 × 100%
= 48%
Therefore, the enantiomeric excess (e.e.) of the mixture containing 5.70 g of (-)-glyceraldehyde and 2.0 g of (+)-glyceraldehyde is 48%.
To know more about enantiomeric excess here
https://brainly.com/question/32199328
#SPJ4
how many molecules are there in 1.5 mol of methane
Answers
Answer: A mole of something means that there are 6.02 X 10^23 of that something. So, a mole of methane molecules is 6.02 X 10^23 methane molecules. So, 1.5 moles of methane will contain 1.5*(6.02 X 10^23) = 9.0 X 10^23 methane molecules
Explanation:
classify matter as elements mixtures and compounds
Answers
Answer:
An element contains just one type of atom. A compound contains two or more different atoms joined together. A mixture contains two or more different substances that are only physically joined together, not chemically. A mixture can contain both elements and compounds.
What is the formal charge on the oxygen atom in the structure below?
a. −
1
.
b. 0
.
c. +
1
.
d. +
2
.
Answers
The difference between the number of valence electrons an isolated atom of that element would have and the number of electrons it actually has in the molecule is defined as the formal charge on that atom in the molecule.
The formal charge is computed as follows:
Formal Charge = (Number of lone pair electrons + Number of bonding electrons / 2) Valence Electrons
The oxygen atom has six valence electrons in the structure you provided, four of which are bonding electrons and two of which are lone pair electrons. The formal charge on the oxygen atom is calculated as follows:
Formal Charge = 6 minus (2 + 4 / 2) = 6 minus (2 + 2) = 6 minus 4 = 2 minus 4 = -2
As a result, the oxygen atom in the structure has a formal charge of -2
To Learn More About oxygen click
https://brainly.com/question/13370320
#SPJ4
Level 1
What is the first step in completing the
scientific method?
A: Experimentation
B: Forming a hypothesis
C: Analyzing data
D: Making observations
Q1
Level 1
Q2
Which of the following is not true about
a hypothesis?
A: It is an explanation for an observation
B: It must restate the question
1. C. It is testable
D. It can be written as an if/then statement,
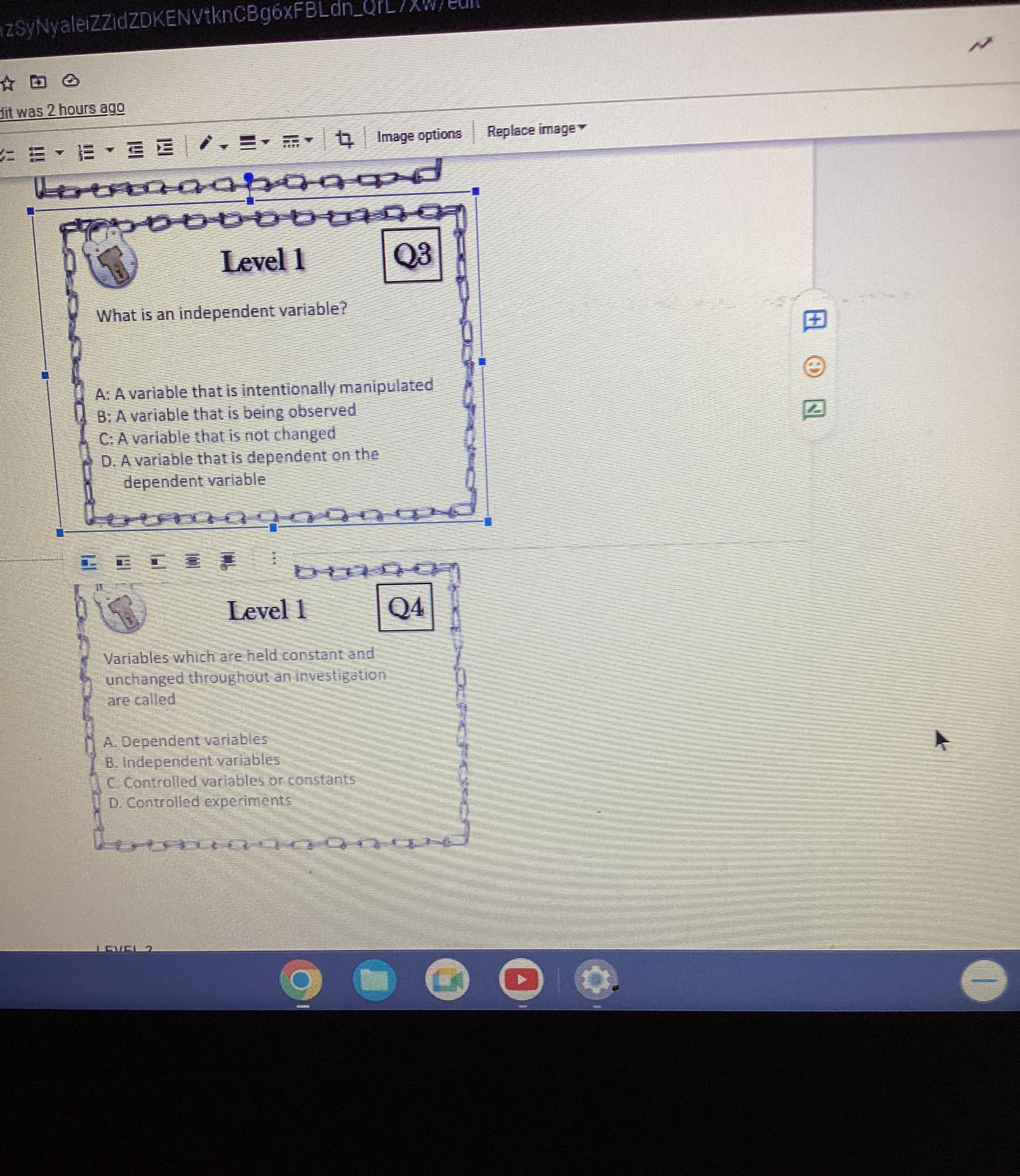
Answers
Answer: Level 1's answer is D/ Making observations. The second one is it must restate the question/ B. No. 3:variable (often denoted by x ) whose variation does not depend on that of another. No.4 IS Controlled Vari
Explanation:
How we can preserve the wetlands areas of nepal?
Answers
Answer:
Reduce, reuse, and recycle your waste and trash. Protecting the environment helps protect the wetlands, especially since trash can make its way into the water. The best and easiest way to protect the environment is by limiting your household waste.
1) 2 C3H7OH + 9 O2 ⇒ 6 CO2 - 8 H2O
If 8 grams of C3H7OH react with 4 grams of O₂
a) What is the limiting reactant? b) What is the excess reactant? c) How many moles of CO₂ can be produced? d) How many grams of CO₂ can be produced? e) How many grams of excess reactant react? f) How many grams of excess reactant remain?
Answers
Answer:
a) To determine the limiting reactant, we need to compare the amount of product that can be produced by each reactant. To do this, we need to convert the mass of each reactant to moles.
For C3H7OH:
moles = mass / molar mass = 8 g / 60.1 g/mol = 0.133 mol
For O2:
moles = mass / molar mass = 4 g / 32 g/mol = 0.125 mol
According to the balanced equation, 2 moles of C3H7OH react with 9 moles of O2 to produce 6 moles of CO2. Therefore, for 0.133 mol of C3H7OH, we need:
moles of O2 = (9/2) x 0.133 mol = 0.597 mol
Since we only have 0.125 mol of O2, it is the limiting reactant. Therefore, O2 is the limiting reactant.
b) C3H7OH is the excess reactant.
c) Using the balanced equation, we can see that 2 moles of C3H7OH react with 9 moles of O2 to produce 6 moles of CO2. Therefore, for 0.125 mol of O2, we can produce:
moles of CO2 = (6/9) x 0.125 mol = 0.083 mol
d) The molar mass of CO2 is 44.01 g/mol. Therefore, the mass of CO2 produced is:
mass of CO2 = moles of CO2 x molar mass of CO2
mass of CO2 = 0.083 mol x 44.01 g/mol = 3.65 g
e) To calculate how much excess reactant is used, we need to first determine how much of the excess reactant is present:
moles of C3H7OH = mass / molar mass = 8 g / 60.1 g/mol = 0.133 mol
moles of C3H7OH needed = (2/9) x moles of O2 = (2/9) x 0.125 mol = 0.028 mol
mol of excess C3H7OH = moles of C3H7OH present - moles of C3H7OH needed
mol of excess C3H7OH = 0.133 mol - 0.028 mol = 0.105 mol
moles of O2 needed = (9/2) x mol of excess C3H7OH = (9/2) x 0.105 mol = 0.473 mol
moles of O2 used = moles of limiting reactant = 0.125 mol
moles of excess C3H7OH used = (1/2) x moles of O2 used = (1/2) x 0.125 mol = 0.0625 mol
f) To calculate how much excess reactant remains, we need to subtract the moles of excess reactant used from the initial moles of excess reactant:
mol of excess C3H7OH remaining = 0.105 mol - 0.0625 mol = 0.0425 mol
The mass of excess reactant remaining can be calculated as follows:
mass of excess C3H7OH remaining = mol of excess C3H7OH remaining x molar mass of C3H7OH
mass of excess C3H7OH remaining = 0.0425 mol x 60.1 g/mol = 2.55
Find the number of grams in 16.95 mol hydrogen peroxide (H2O2). Round your
answer to two decimal places and be sure to include the proper units.
Answers
Answer: There are 576.46 number of grams present in 16.95 mol hydrogen peroxide \((H_{2}O_{2})\).
Explanation:
Number of moles is defined as the mass of substance divided by its molar mass.
The molar mass of \(H_{2}O_{2}\) is 34.01 g/mol. Hence, mass of hydrogen peroxide present in 16.95 moles is calculated as follows.
\(Moles = \frac{mass}{molarmass}\\16.95 mol = \frac{mass}{34.01 g/mol}\\mass = 576.46 g\)
Thus, we can conclude that there are 576.46 number of grams present in 16.95 mol hydrogen peroxide \((H_{2}O_{2})\).
Which equation is a decomposition reaction? Li2CO3 Right arrow. Li2O CO2 Zn HCl Right arrow. ZnCl2 H2 Na2O CO2 Right arrow. Na2CO3 C6H12O6 O2 Right arrow. CO2 H2O.
Answers
The equation that has been the representation of the decomposition reaction has been \(\rm Li_2CO_3\;\rightarrow\;Li_2O\;+\;CO_2\).
The chemical reaction has been defined as the reaction in which the chemical structure, composition and properties of the substances changes.
The chemical reaction has been given as the synthesis, decomposition and displacement reaction.
Decomposition reactionThe chemical reaction in which there has been formation of a new product has been the synthesis reaction. The reaction in which the reactant has been breakdown into the constituent substances has been the decomposition reaction.
The reaction in which the less reactive element has been displaced by the more reactive element has been the displacement reaction.
The following reactions have been categorized as:
\(\rm Li_2CO_3\;\rightarrow\;Li_2O\;+\;CO_2\)The reactant has been broken down into products. It has been a decomposition reaction.
\(\rm Zn\;+\;HCl\;\rightarrow\;ZnCl_2\;+\;H_2\)There has been replacement of H by Zn. Thus, it has been a displacement reaction.
\(\rm Na_2O\;+\;CO_2\;\rightarrow\;Na_2CO_3\)The reactants combine to form a new product. Thus, it has been a synthesis reaction.
\(\rm C_6H_1_2O_6\;+\;O_2\;\rightarrow\;CO_2\;+\;H_2O\)The reaction has been the breakdown of glucose in the presence of oxygen. It has been a degradation reaction.
Thus, the equation that has been the representation of the decomposition reaction has been \(\rm Li_2CO_3\;\rightarrow\;Li_2O\;+\;CO_2\).
Learn more about decomposition reaction, here:
https://brainly.com/question/24936069
Which equation is a decomposition reaction?
A. Li2CO3 Right arrow. Li2O + CO2
A ingle replacement reaction i run in the lab between 45. 0g hydrofluoric acid,HF, and 125. 0g tin forming tin(ll) fluoride and another product
Answers
The molar mass of tin(II) chloride is: 84.9 g
Calculate the molar mass of tin(II) chloride?
The number of moles n1 is thus:
n1= m1/M1 = 2.25 mol
According to the balanced reaction, the ratio by number of moles n of tin(II) chloride produced to the number of moles n1 of HF used is 1:2
As such, the number of moles of tin(II) chloride produced is :
n = 1/2 \(n_{1}\) = 0.45 mol
The molar mass of tin(II) chloride is:
M= A\(_{r}\)(Sn)+2A\(_{r}\)(Cl) = 188.7 g/mol
The mass m produced is thus:
m = nM
= 0.45 mol × 188.7 g/mol
≈ 84.9 g
Therefore, the molar mass of tin(II) chloride is: 84.9 g.
To know more about molar mass, check out:
https://brainly.com/question/837939
#SPJ4
Find the quantinum numbers n,m,l,s for the last of potassium layer pleasee help explain correctly all
Answers
Answer:
Quantum numbers of the outermost electron in potassium:
\(n = 4\).\(l = 1\).\(m_l = 0\).Either \(m_s = 1/2\).Explanation:
Refer to the electron configuration of a potassium atom. The outermost electron in a ground-state potassium atom is in the \(4s\) orbital (fourth \(s\) orbital.)
The quantum number \(n\) (the principal quantum number) specifies the main energy shell of an electron. This electron is in the fourth main energy shell (as seen in the number four in the orbital.) Hence, \(n = 4\) for this electron.
The quantum number \(l\) (the angular momentum quantum number) specifies the shape (\(s\), \(p\), \(d\), etc.) of an electron. \(l = 1\) for \(s\!\) orbitals (such as the one that contains this electron.
Quantum numbers \(n\) and \(l\) specify the shape of an orbital. On the other hand, the magnetic quantum number \(m_l\) specifies the orientation of these orbitals in space.
However, \(s\) orbitals are spherical. Regardless of the value of \(n\), the only possible \(m_l\) value for electrons in \(s\!\) orbitals is \(m_l = 0\).
The spin quantum number \(m_s\) distinguishes between the two electrons in an orbital. The two possible values of \(m_s \!\) are \((+1/2)\) and \((-1/2)\). Typically, the first electron in an orbital is assigned an upward (\(\uparrow\)) spin, which corresponds to \(m_s = (+1/2)\).
Consider the intermediate chemical reactions. 2 equations. First: upper C a (s) plus upper C upper O subscript 2 (g) plus one half upper O subscript 2 (g) right arrow upper C a upper C upper O subscript 3 (s). Delta H 1 equals negative 812.8 kilojoules. Second: 2 upper C a (s) plus upper O subscript 2 (g) right arrow 2 upper C a upper O (s). Delta H 2 equals negative 1, 269 kilojoules. The final overall chemical equation is Upper Ca upper O (s) plus upper C upper O subscript 2 (g) right arrow upper C a upper C upper O subscript 3 (s).. When the enthalpy of this overall chemical equation is calculated, the enthalpy of the second intermediate equation is halved and has its sign changed. is halved. has its sign changed. is unchanged.
Answers
Answer: When the enthalpy of this overall chemical equation is calculated, the enthalpy of the second intermediate equation is halved and has its sign changed.
Explanation:
Hess’s law of constant heat summation states that the amount of heat absorbed or evolved in a given chemical equation remains the same whether the process occurs in one step or several steps.
According to this law, the chemical equation is treated as ordinary algebraic expressions and can be added or subtracted to yield the required equation. This means that the enthalpy change of the overall reaction is equal to the sum of the enthalpy changes of the intermediate reactions.
The overall chemical reaction follows:
\(CaO(s)+CO_2\rightarrow CaCO_3(s)\) \(\Delta H^o_{rxn}=?\)
The intermediate balanced chemical reaction are:
(1) \(Ca(s)+CO_2(g)+\frac{1}{2}O_2(g)\rightarrow CaCO_3(s)\) \(\Delta H_1=-812.8kJ\)
(2) \(2Ca(s)+O_2(g)\rightarrow 2CaO(s)\) \(\Delta H_2=-1269kJ\)
The expression for enthalpy of the reaction follows:
\(\Delta H^o_{rxn}=[1\times (\Delta H_1)]+[\frac{1}{2}\times (-\Delta H_2)]\)
Hence, when the enthalpy of this overall chemical equation is calculated, the enthalpy of the second intermediate equation is halved and has its sign changed.
Answer:
A. is halved and has its sign changed.
Explanation:
just took the test on edge
Physical and chemical properties are used to describe and identify matter. Physical properties can be observed without changing the identity of the substance. Chemical properties can be observed by attempting to change the identity of a substance. What are two physical and chemical properties that you can use to help you identify a substance?
Answers
Extensive properties, such as mass and volume, depend on the amount of matter that is being measured. ... Both extensive and intensive properties are physical properties, which means they can be measured without changing the substance's chemical identity
This is the process that cells reproduce and replace old or damaged cells.
Question 5 options:
Mitosis
Meiosis
Ribosomes
Cytokinesis
Answers
Didnndxbdbdn
Uranium has three isotopes with the following percent abundances: 234- (0.0058%), 235- (0.71%), 238- (99.23%). Without doing any calculations, what do you expect the atomic mass of uranium to be in whole numbers. Why?
Answers
Answer:
Because it is the same element but has different atomic mass
An element with a mass number of 11 and an atomic number of 5 has how many
neutrons?
Answers
Answer:
6 neutrons
Explanation:
6 neutrons
Boron having an atomic number of 5 means that it will have 5 protons. 11 atomic mass units in total. Neutrons also have a atomic mass unit of 1. So there are 6 neutrons
what is the smallest number of ice cubes at 0 c, each containing one mole of water, necessary to cool 800.0 g of liquid water initially at 20c to 0c
Answers
As each ice cube contains one mole of water, you would need at least 44.4 ice cubes to cool the liquid water from 20°C to 0°C
To solve this problem, we need to use the equation:
Q = m × c × ΔT
where Q is the amount of heat transferred, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
We can first calculate the amount of heat that needs to be transferred from the liquid water to the ice cubes:
Q = m × c × ΔT
Q = 800.0 g × 4.184 J/g°C × (-20°C)
Q = -67,072 J
The negative sign indicates that heat is leaving the liquid water and being absorbed by the ice cubes.
Next, we need to determine how many moles of water are in 800.0 g of liquid water:
n = m/M
n = 800.0 g ÷ 18.015 g/mol
n = 44.4 mol
Therefore, we need at least 44.4 ice cubes, each containing one mole of water, to cool the liquid water from 20°C to 0°C. However, this assumes that all of the heat transferred from the liquid water is used to melt the ice cubes, and none of it is lost to the environment. In reality, we would need more ice cubes to account for any heat loss.
Learn more about heat here:https://brainly.com/question/1429452
#SPJ11