Sublimation C
Melting E
Condensation D
Freezing A
Depostion F
Vaporization B
Please help me Match it
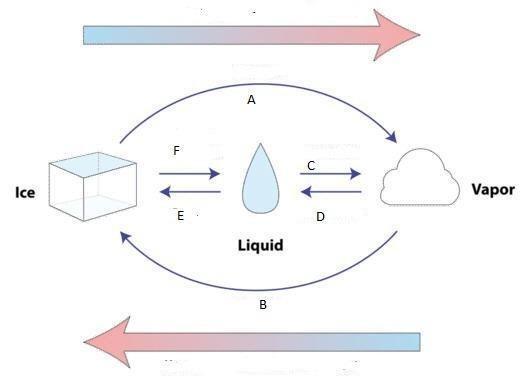
Answers
Answer:
A is Vaporisation
B is Sublimation
C is condensation
D is Deposition
E is Freezing
F is melting
Related Questions
A chemist is trying to classify an unknown substance as either a metal or nonmetal. What question should the chemist use to help classify the material?
A. Does the material feel hard to the
touch?
B. Does the material feel rough or smooth?
C. Is the material a good conductor or a poor conductor?
D. Will the material float in water?
Please Help me!!!
Answers
Answer:
c
Explanation:
metals are good conductors, while non metals are not good conductors
What is the only natural way that carbon can move OUT of the geosphere?
burning of fossil fuels
volcanic eruptions
dissolution
decomposition
Answers
Answer:
volcanic eruptions
Explanation:
True or False:
A compound contains 38.7% C, 9.70% H, and 51.6% O. Thus
the empirical formula for the compound is CH2O.
Answers
yes sure, true it can beee
Answer:
False
Explanation:
answers for both boxes please

Answers
Explanation:
\( \frac{6.900 \times {10}^{10} }{4.000 \times {10}^{8} } \\ 1.725 \times {10}^{10 - 8} \\ 1.725 \times {10}^{2} \)
Hope it will help you :)
It took 20.0 mL of NaOH to neutralize 25 mL of 0.50 M HCl. What is the molarity of the base?
Answers
Answer:
5/8 M
Explanation:
NaOH + HCl ====> NaCl + H2O
in 25 mL of 0.5M acid, we have 1/80 Mol of HCl
it mean we must have 1/80 Mol of NaOH.
20 mL = 1/50 L
(1/80)Mol / (1/50)L = 5/8 M
what is a salient factor?
Answers
Answer:
technology is a silent factor
Explanation:
learned about it in computer class.
What best describes what models are used for?
A. Models are used as substitutes for compounds.
B. Models are used to explain how something works.
C. Models are used to perform experiments.
D. Models are used to develop new technologies.
Answers
Explanation:
is b or c but i think asnwer is b
The correct statement is " Models are used to explain how something works."
What is model?To introduce specific content, models might be employed. A model can help students learn crucial words and create a setting in which they can investigate pertinent processes.
Models can be used to improve explanations, promote discussion, make predictions, present graphical images of abstract concepts, and generate mental models in research.
Therefore, Models are used to explain how something works .
To know more about models.
https://brainly.com/question/14281845.
#SPJ2
Calculate the volume of 3.00 moles of CH4 at 25°C and 1.5 atm.
Answers
The volume of methane is calculated by using ideal gas law equation (pv=nRT) and the volume of methane is 4.95L.
The volume (V) occupied by the n moles of any gas has pressure(P) and temperature (T) Kelvin, the relationship for these variables PV=nRT where R gas constant is called the ideal gas law.
Formula used :- pv= nRT.
where, p= pressure
v= volume,
n= number of moles,
R = gas constant,
T= temperature.
Given:- p= 1.5 atm, n= 3, R= 8.314, T= 298K, v=?
By putting values in the formula we will get:-
pv=nRT, or v=\(\frac{nRT}{p} = \frac{3*8.314*298}{1.5} = 4,955.14ml= 4.95L.\)
Hence, the volume of methane is 4.95L.
To know more about ideal gas law refer to:-
https://brainly.com/question/27870704
#SPJ4
differentiate between edible and non edible mushroom
Answers
Edible mushrooms: Consuming edible mushrooms is safe and provides health advantages like fiber, vitamins, and minerals.
Examples: Button mushrooms.Non-Edible mushrooms: Mushrooms that cannot be eaten could be harmful or have unappealing flavors and textures that could be harmful if consumed.
Examples: Death Cap.
are essential nutrients needed for tissue growth and repair.
Vitamins
water
fats
proteins
Answers
hope this helped:)
Answer
Proteins
Explanation:
edg.2020
what is a similar math problem to the calcium and water equation
Answers
Answer: Calcium is a metal and a typical reaction of a metal with water will be as follows: ... the chemical reaction for calcium and water will be: [Word Equation] Calcium + 48.
Explanation: Please Please mark me as brainliest!
PLEASE HELP!!!!! I'LL YOU BRAINIEST!!!!



Answers
Answer:
I believe 11 is B 12 is C 13 is B and 14 is C
how is it that a class of compounds as chemically inert as the cfcs can pose an environmental problem like the destruction of the ozone layer?
Answers
The class of compounds known as chlorofluorocarbons (CFCs) are chemically inert, meaning they are not easily reactive with other substances. However, they can still pose an environmental problem, particularly in relation to the destruction of the ozone layer.
The issue with CFCs lies in their stability and their ability to reach the upper atmosphere. CFCs are extremely stable compounds, meaning they do not break down easily under normal conditions. When released into the atmosphere, they can persist for a long time without undergoing significant degradation. This stability allows them to gradually rise to the stratosphere, where the ozone layer is located.
Once in the stratosphere, CFCs can undergo photodissociation due to the absorption of ultraviolet (UV) radiation. This process releases chlorine atoms, which can then participate in catalytic reactions that destroy ozone molecules. Chlorine acts as a catalyst in the destruction of ozone, meaning it is not consumed in the process and can continue to deplete ozone molecules.
In summary, although CFCs are chemically inert, their stability and ability to reach the stratosphere enable them to cause ozone layer depletion. Once in the upper atmosphere, CFCs release chlorine atoms that act as catalysts in the destruction of ozone, leading to the environmental problem of ozone layer depletion. The stability of CFCs allows them to persist and continue to contribute to ozone depletion over extended periods of time.
To learn more CFCs click here:
brainly.com/question/31732903
#SPJ11
What type of bonding is present in magnesium nitrate, Mg(NO3)2 ? *
O ionic
O covalent
O covalent and metallic
O
ionic and covalent
Answers
Answer:
D
Explanation: I guess
Help with theses two different problems!
1.) 125mL of what is added to 45.3mL of 0.71m NaOH solution
2.) 550mL of water is added to 125mL of 3.01M KOH solution
Answers
1. the final concentration of NaOH after adding 125 mL of water to 45.3 mL of 0.71 M NaOH solution is approximately 0.189 M.
2. the final concentration of KOH after adding 550 mL of water to 125 mL of 3.01 M KOH solution is approximately 0.557 M.
1.) If 125 mL of water is added to 45.3 mL of a 0.71 M NaOH solution, the resulting solution will be a diluted NaOH solution. The addition of water will increase the total volume while reducing the concentration of NaOH. To determine the final concentration of NaOH, we need to consider the conservation of moles.
First, let's calculate the moles of NaOH in the initial solution:
moles of NaOH = volume (in L) × concentration (in M)
moles of NaOH = 0.0453 L × 0.71 M = 0.0321433 moles
After adding 125 mL (0.125 L) of water, the total volume of the solution becomes 0.0453 L + 0.125 L = 0.1703 L.
To find the final concentration, we divide the moles of NaOH by the total volume:
final concentration of NaOH = moles of NaOH / total volume
final concentration of NaOH = 0.0321433 moles / 0.1703 L ≈ 0.189 M
Therefore, the final concentration of NaOH after adding 125 mL of water to 45.3 mL of 0.71 M NaOH solution is approximately 0.189 M.
2.) If 550 mL of water is added to 125 mL of a 3.01 M KOH solution, the resulting solution will also be a diluted solution. Again, we will apply the conservation of moles to determine the final concentration of KOH.
First, calculate the moles of KOH in the initial solution:
moles of KOH = volume (in L) × concentration (in M)
moles of KOH = 0.125 L × 3.01 M = 0.37625 moles
After adding 550 mL (0.55 L) of water, the total volume of the solution becomes 0.125 L + 0.55 L = 0.675 L.
To find the final concentration, divide the moles of KOH by the total volume:
final concentration of KOH = moles of KOH / total volume
final concentration of KOH = 0.37625 moles / 0.675 L ≈ 0.557 M
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
Creepers! A creepy corpse stole
all of Ma Ribbins money from her
grocery store. The crime scene
team found a compound that was
27.37%Na, 1.20%H, 14.30% C, and
57.14%0. What was the empirical
formula of this compound, and
who committed the theft?
Answers
Thus, the empirical formula of this compound is S2O5 S 2 O 5 .
An empirical formula is what GCSE?The simplest whole number ratio of atoms from each element in a compound is called the empirical formula. The precise amount of atoms of each element in a compound is given by its molecular formula. the connection between the molecular formula and empirical formula.
The simplest whole number ratio of atoms from each component of a compound is its empirical formula. It is determined empirically utilizing data from trials. For instance, whereas the empirical formula for glucose is CH 2O, its molecular formula is C 6H 12O 6.
learn more about empirical formula
https://brainly.com/question/1603500
#SPJ1
1. What element is a metalloid in group 3?
2. What group 1 element is a NON metal?
3.what period 3 element has 8 valence electrons
Answers
Answer:
1.The metalloids; boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), polonium (Po) and astatine (At) are the elements found along the step like line between metals and non-metals of the periodic table.
2.hydrogen
The elements generally classified as nonmetals include one element in group 1 (hydrogen);
3.element iron
The element iron is in group 8, and therefore has two or three apparent valence electrons.
NEED ASAP. WILL MARK BRAINLIEST!
1. (06.01 LC)
Based on the kinetic theory, which statement is true? (15 points)
A. The particles of matter are stationary.
B. Matter is composed of very small particles.
C. Matter is made up of only charged particles.
D. The particles of matter have zero kinetic energy.
Answers
Answer:
B. Matter is composed of very small particles.
Explanation:
All matter is composed of extremely small particles called atoms. All atoms of a given element are identical in size, mass,
The statement is true based on the kinetic theory is that; "matter is composed of very small particles.".
Kinetic theory of matter
The kinetic theory of matter holds that the particles that compose matter are in constant random motion. In this theory, all matter is composed of molecules.
Now, the statement is true based on the kinetic theory is that; "matter is composed of very small particles.".
Learn more about kinetic theory:https://brainly.com/question/14349214?
#SPJ2
What will make an atom without an electrical charge? (1 point) Responses An equal number of neutrons and electrons, because neutrons are positively charged and electrons are negatively charged. An equal number of neutrons and electrons, because neutrons are positively charged and electrons are negatively charged. An equal number of protons and electrons, because electrons are positively charged and protons are negatively charged. An equal number of protons and electrons, because electrons are positively charged and protons are negatively charged. More electrons than protons, because electrons are negatively charged and protons are positively charged, and electrons are much smaller than protons More electrons than protons, because electrons are negatively charged and protons are positively charged, and electrons are much smaller than protons An equal number of protons and electrons, because electrons are negatively charged and protons are positively charged.
Answers
An equal number of protons and electrons, because electrons are negatively charged and protons are positively charged, and their charges cancel out when the numbers are equal.
An atom consists of positively charged protons, negatively charged electrons, and neutral neutrons. The number of protons in the nucleus determines the atomic number and the element that the atom belongs to. The number of electrons orbiting the nucleus is equal to the number of protons in a neutral atom. Therefore, an equal number of protons and electrons will result in a neutral atom without an electrical charge.
If the number of electrons is greater than the number of protons, the atom will have a negative charge, and if the number of protons is greater than the number of electrons, the atom will have a positive charge. The equal number of protons and electrons in a neutral atom creates an electrically balanced state, where the attractive forces between the positive and negative charges are equal, resulting in no net electrical charge.
The complete question is
What will make an atom without an electrical charge? (1 point)
Responses
An equal number of neutrons and electrons, because neutrons are positively charged and electrons are negatively charged.An equal number of protons and electrons, because electrons are positively charged and protons are negatively charged. More electrons than protons, because electrons are negatively charged and protons are positively charged, and electrons are much smaller than protonsAn equal number of protons and electrons, because electrons are negatively charged and protons are positively charged.To know more about the Atom, here
https://brainly.com/question/31942139
#SPJ1
What is the number of carbon atoms in a cycloalkyne with 8 hydrogen atoms
Answers
Answer:
4
Explanation:
C4H8
All molecules have the same kinetic energy and hence the same speed.
O True
O False
Answers
uppose some solid calcium hydroxide is inadvertently transferred along with the saturated liquid for analysis. a) will more, less, or the same amount of hydrochloric acid be used for the analysis in part a? explain. b) how will the molar solubility be affected? explain. c) how will the solubility product constant k sp be affected? explain
Answers
The additional calcium hydroxide will react with some of the hydrochloric acid, leading to a higher consumption of the acid for the analysis.
If solid calcium hydroxide is inadvertently transferred along with the saturated liquid for analysis, the following effects can be expected:
a) More hydrochloric acid will be used for the analysis in part a. This is because calcium hydroxide reacts with hydrochloric acid to form calcium chloride and water according to the following equation:
Ca(OH)₂ + 2HCl → CaCl₂ + 2H₂O
b) The molar solubility of calcium chloride will decrease due to the additional calcium hydroxide. This is because calcium hydroxide reacts with calcium chloride in the solution to form calcium carbonate, which is insoluble in water:
CaCl₂ + Ca(OH)₂ → CaCO₃ + 2H₂O
As a result, more calcium carbonate will precipitate out of the solution, leading to a decrease in the molar solubility of calcium chloride.
c) The solubility product constant (Ksp) of calcium hydroxide will increase due to the additional solid. This is because the presence of more calcium hydroxide will increase the concentration of calcium and hydroxide ions in the solution, shifting the equilibrium towards the formation of more solid calcium hydroxide. This will increase the value of Ksp, indicating a higher degree of saturation of the solution with respect to calcium hydroxide.
Learn more about solubility here
https://brainly.com/question/31493083
#SPJ11
Which of these is not a component of Rutherford’s model of the atom?
Answers
The Rutherford's model lacks an atom's electrical structure and electromagnetic radiation.
What elements make up Rutherford's atomic model?According to the idea, an atom has a tiny, compact, positively charged center called a nucleus, where almost all of the mass is concentrated, while light, negatively charged particles called Like planets circle the Sun, electrons also travel a great distance around it. Rutherford discovered that an atom's interior is mostly empty.
What does Rutherford's conclusion leave out?Rutherford's alpha scattering experiment did not come to any conclusions on how quickly positively charged particles travel. The nucleus, or core, of the atom contains the positively charged particles.
To know more about Rutherford's model visit:-
https://brainly.com/question/11749615
#SPJ1
What is the relationship between the concentration and the rate of the reaction?
negative
neutral
positive
Answers
Answer:
When the concentration of all the reactants increases, more molecules or ions interact to form new compounds, and the rate of reaction increases. When the concentration of a reactant decreases, there are fewer of that molecule or ion present, and the rate of reaction decreases.
Answer:
Negative
Explanation:
State with reason in each case whether the PH would increase, decrease or remain constant if the following experiments were carried out. (i) neutralizing bench HNO3 (ii) diluting 25.0cm3 of a given NaOH solution to 100.0cm3 (iii) concentrating a solution of NaCl
Answers
(i) The pH would decrease if bench \(HNO_{3}\) is neutralized.
(ii) The pH would increase if 25.0 cm3 of a given NaOH solution is diluted to 100.0 cm3.
(iii) The pH would remain constant if a solution of NaCl is concentrated.
HNO3 is a strong acid that dissociates completely in water to form H+ ions. When \(HNO_{3}\) is neutralized, it reacts with a base to form a salt and water. Since \(HNO_{3}\) is an acid, the addition of a base would reduce the concentration of H+ ions in the solution, resulting in a decrease in the overall acidity. As a result, the pH of the solution would increase.
NaOH is a strong base that dissociates completely in water to form OH- ions. When the NaOH solution is diluted, the concentration of OH- ions decreases while the volume of the solution increases. Since pH is a measure of the concentration of H+ ions in a solution, a decrease in the concentration of OH- ions would lead to an increase in the concentration of H+ ions, making the solution more acidic. Consequently, the pH of the solution would increase.
NaCl is a neutral salt that does not undergo hydrolysis in water, meaning it does not release or accept H+ or OH- ions. Concentrating the solution does not alter the nature of the ions present in the solution or their concentrations. Therefore, the concentration of H+ and OH- ions remains unchanged, resulting in a constant pH.
for such more questions on solution
https://brainly.com/question/25326161
#SPJ8
convert 5 mole(s) of Xe into molecules
Answers
Answer:
5 mol Xe × 6.02×10^23
------------ ----------------
1 1 mol Xe
3.01x10^24
I would calculate on your calculator to make sure
if 17.9 mlml of 0.800 m hcl solution are needed to neutralize 5.00 mlml of a household ammonia solution, what is the molar concentration of the ammonia?
Answers
The molar concentration of the ammonia 2.86 M.
Balanced reaction is given as :
HCl + NH3 = NH4Cl
so using the formula : M1V1 = M2V2
M1 = 0.800 M
V1 = 17.9 mL
V2 = 5.00 mL
putting values :
0.800 M x 17.9 mL = M2 x 5.00 mL
M2 = 2.86 M
To balance chemical equations, stoichiometric coefficients are added to the reactants and products. This is significant because a chemical equation must obey the laws of conservation of mass and constant proportions, which require that the same number of atoms of each element exist on the reactant and product sides of the equation.
To learn more about molar concentration visit:https://brainly.com/question/15532279
#SPJ4
Learning about the parts of an atom is important because
It can help us create a better model of an atom.
It can help us create new elements.
It can help us study how cells in the body functions.
It can help us explain the universe.
Answers
Answer:
"it can help us explain the universe" or "it can help us study how cells in the body function"
Explanation:
you choose which one you want but their still both technicly right
Classify the chemical reaction: Cl₂O5 + H₂O → 2HCIO3
Answers
The reaction is an addition reaction.
What is an addition reaction?Addition reactions are chemical reactions involving 2 or more reactants reacting to produce a single product.
Addition reactions are different from decomposition reactions. In the latter, a single reactant decomposes to give two or more products.
In the illustrated reaction, Cl₂O5 and H₂O react together to form a single product, HCIO3.
More on addition reactions can be found here: https://brainly.com/question/13669873
#SPJ1
Calculate the H+ ion concentration in a 8.8 x 10-4 M Ca(OH)2
solution.
Answers
form the molar concentration of the Ca(OH)2 which is a base we can deduct the pOH
pOH=-lg[OH-]
[OH-]=8.8 x 10-4 M
pOH=4
pH=14-pOH
pH=10
-lg[H+]=10
[H+]=10^-10
The concentration of Hydrogen ion [H⁺] in 8.8×10¯⁴ M Ca(OH)₂ is 5.68×10¯¹² M
The concentration of Hydrogen ion [H⁺] in a solution talks about the acidicity the solution. Thus, we can obtain the concentration of Hydrogen ion [H⁺] in 8.8×10¯⁴ M Ca(OH)₂ solution as illustrated below:
Step 1:Data obtained from the question
Concentration of Ca(OH)₂ = 8.8×10¯⁴ M
Concentration of Hydrogen ion [H⁺] =..?
Step 2:Determination of the concentration of Hydroxide ion [OH¯]
Concentration of Ca(OH)₂ = 8.8×10¯⁴ M
Concentration of Hydroxide ion [OH¯] =?
Ca(OH)₂ (aq)⇄Ca²⁺ (aq) + 2OH¯(aq)
From the balanced equation above,
1 mole Ca(OH)₂ produced 2 moles of OH¯.
Therefore, 8.8×10¯⁴ M Ca(OH)₂ will produce = 8.8×10¯⁴ × 2 = 1.76×10¯³ M
Thus, the Concentration of Hydroxide ion [OH¯] is 1.76×10¯³ M
Step 3:Determination of concentration of Hydrogen ion [H⁺]. Concentration of Hydroxide ion [OH¯] = 1.76×10¯³ M
Concentration of Hydrogen ion [H⁺] =..?\(H^{+} * OH^{-} = 1*10^{-14}\\H^{+} * 1.76^{-3} = 1*10^{-14}\)
Divide both side by 1.76×10¯³
\(H^{+} = \frac{1*10^{-14} }{1.76*10^{-3}}\\\)
[H⁺] = 5.68×10¯¹² M
Therefore, the concentration of Hydrogen ion [H⁺] in the solution is 5.68×10¯¹² M
Learn more: https://brainly.com/question/17122769