Studies have found that certain chemicals that help repair damaged cells only function while we sleep. What theory best explains this?A. restorative theory of sleep
B. REM sleep
C. sleep walking
D. the brain stem is not yet fully mature
Answers
Restorative Theory of Sleep is the best theory to explain this. This theory proposes that our bodies use sleep to help repair and restore cells, tissues, and organs.
During sleep, the body is able to produce growth hormones, repair damaged cells, and regenerate tissue. It also helps to regulate the immune system and restore energy levels. This theory explains why certain chemicals that help repair damaged cells only function while we sleep. This theory states that sleep helps restore the body and mind from the wear and tear of daily life, allowing the body to repair itself. This includes the repair of damaged cells, which can only happen while we sleep.
To learn more about energy click here https://brainly.com/question/626780
#SPJ4
Related Questions
186km at 3:00 the speed limit is 65km/h
Answers
He will arrive at the second city on time
Using the formula for calculating speed expressed as:
Distance = Speed * time
Given the following parameters
Speed = 65km/hr
Time = 3 hours (from noon to 3PM)
Get the distance covered by Isaiah;
Distance = 65 * 3
Distance = 195km > 186km
Since the distance covered by Isaiah is greater than the distance from one city to another city, hence he will arrive at the second city on time
What is Speed?
The speed at which an object's location changes in any direction. The distance traveled in relation to the time it took to travel that distance is how speed is defined. Since speed simply has a direction and no magnitude, it is a scalar quantity.
Therefore,
186km at 3:00 the speed limit is 65km/h
He will arrive at the second city on time
Using the formula for calculating speed expressed as:
Distance = Speed * time
Given the following parameters
Speed = 65km/hr
Time = 3 hours (from noon to 3PM)
Get the distance covered by Isaiah;
Distance = 65 * 3
Distance = 195km > 186km
To learn more about Speed from the given link:
https://brainly.com/question/13943409
#SPJ4
How much more average Kinetic Energy do molecules have at 50°C compared to 25°C?
Answers
The kinetic energy is 0.5177 × 10⁻²¹ J more at 50°C compared to 25°C.
The average kinetic energy of a molecule is directly proportional to the absolute temperature of a gas.
KE = ( 3/2 ) ( R / Nₐ ) T
Where T is the temperature of the molecule, R is the gas constant, and Nₐ is Avogadro's number.
Now, R = 8.314 J/mol.K
Avogadro's number, Nₐ = 6.022 × 10²³ atoms/ mol
The average kinetic energy at 50° C is:
T = 50° C = 323 K
KE₁ = ( 3/2 ) × ( R / Nₐ ) × T₁
KE₁ = ( 3 × 8.314 × 323 ) / ( 2 × 6.022 × 10²³ )
KE₁ = 668.90 × 10⁻²³ J
KE₁ = 6.6890 × 10⁻²¹ J
The average kinetic energy at 25°C is:
KE₂ = ( 3/2 ) × ( R / Nₐ ) × T₂
KE₂ = ( 3 × 8.314 × 298 ) / ( 2 × 6.022 × 10²³ )
KE₂ = 617.13 × 10⁻²³ J
KE₂ = 6.1713 × 10⁻²¹ J
Now,
The average kinetic energy of the molecules at 50° C compared to 25° C is:
KE = KE₁ - KE₂
KE = 6.6890 × 10⁻²¹ - 6.1713 × 10⁻²¹
KE = 0.5177 × 10⁻²¹ J
Hence, the average kinetic energy is 0.5177 × 10⁻²¹ J more at 50° C compared to 25° C.
Learn more about kinetic energy here:
https://brainly.com/question/8101588
#SPJ9
what is the number of joules needed to increase the temperature of 50.0 grams of water 15°C?
i was also wondering if you could share the steps on how you did it so i can understand how to really do the equation for other questions like this as well.
Answers
Answer:
What occurs when the temperature of 10.0 grams of water is changed from 15.5°C to 14.5°C? [Specific Heat of Water = 4.18 J/g •k] 1) The water absorbs 41.8 joules. 2) The water absorbs 155 joules.
Explanation:
Take a look at the specific heat of water. As you know, a substance's specific heat tells you how much heat is needed in order to increase the temperature of 1 g of that substance by 1∘C . In water's case, you need to provide 4.18 J of heat per gram of water to increase its temperature by 1∘C .
An aluminum ion has 13 protons, 14 neutrons, and 10 electrons. What is the charge of the aluminum ion?
Answers
Answer:
the charge of the aluminum ion is +3
formula of sodium chloride, sodium trioxocarbonate (IV), aluminum oxide, ammonium sulphide, zinc hydroxide, zinc tetraoxosulphate (IV), sodium tetraoxosulphate (VI), trioxonitrate (V) acid, water
Answers
Answer:
The correct answer is Na2CO3. 10H2O
Write a chemical equation showing Bromine reacting with Hydrogen Disulfide to produce Sulfur and
Hydrogen Monobromide (hydrobromic acid).
Answers
Answer:
Br2 + H2S => 2HBr + S
Explanation:
Br2 + H2S => 2HBr + S
Br = 2
H = 2
S = 1
Which of the following does not describe a physical property of iron?
A
Iron is silvery-white or gray in color.
B
Iron has a boiling point of about 3,000°C.
C
Iron is a magnetic element.
D
Iron and sulfur react to form iron sulfide.
Answers
Answer:
B
Explanation:
Iron becomes liquid when we heat it to a temperature of 1535° C this is it's melting point. If we further heat the liquid to 3,000° C it boils; iron is a gas above this temperature.
Which of the following is NOT a correct conversion factor?
1 cm = 0.01 m
1 km = 1000 m
1 cm = 100 m
1 dm = 0.1 m

Answers
Answer: 1 cm = 100m
Explanation: Centi- is the prefix used to designate 0.01 (one hundredth) of something. 1 cm means 0.01 m.
1 cm = 100 m is NOT a correct conversion factor. Option C is correct.
what are conversion factors?The conversion factors are the factors that re-use to convert some values from unit to unit of another value like from centimeter to meter from meter to kilometer from milligram to gram.
In one centimeter it is the suffix used to denote it is the one by hundred value of the on meter or which is equal t one meter so, 1cm = 0.01 meters as it is the 1/100 part f the one-meter distance.
Therefore, Option C is correct. 1 cm = 100 m is NOT a correct conversion factor.
Learn more about conversion factors, here:
https://brainly.com/question/28366871
#SPJ2
Although polymers can be melted at very low temperatures, it is very difficult for polymers to form complex shapes upon cooling. Group of answer choices True False
Answers
Answer:
False
Explanation:
Although polymers can be melted at very low temperatures, it is very difficult for polymers to form complex shapes upon cooling. The statement is false.
What are polymers ?A polymer is a macromolecule, combination of many subunits, it can be found all around us, In DNA naturally occurring biopolymer are present, polypropylene used as the plastic.
Polymers naturally found in plants and animals or may be man-made or called as synthetic polymers.
Different polymers have both physical and chemical properties, like the tensile strength increase due to the chain length and increase in cross-linking, do not melt, present in crystalline to semi-crystalline form.
The polymer is formed by hydrogen bonding and ionic bonding resulting in better cross-linking strength, Dipole-dipole bonding side chains is observed which show its high flexibility, Van der Waals forces can also link the chains.
Learn more about polymers, here:
https://brainly.com/question/17638582
#SPJ2
suppose that the power used by a light bulb in a circuit is 16 W, and the bulb has a resistance of 4 ohms. Calculate the current (in amps) flowing through it.
Answers
Answer:
2 A
Explanation:
The power flowing in a circuit is given by;
P= I^2 R
Where;
I= current = the unknown
R= resistance= 4 ohms
P= power=16 W
I= √P/R
I= √16/4
I= 2 A
1. 547 grams of hydrated mgso4 is heated in a crucible. After heating, 0. 7554 grams of anhydrous mgso4 remains in the crucible. How many waters of hydration were attached to the mgso4?.
Answers
7 waters of hydration were attached to the MgSO4.
1. 547 grams of hydrated mgso4 is heated in a crucible.
molecular weight of mgso4= 120 g/mol
let, x number of water molecule present in this hydrated mgso4
then, molecular weight = 18x +120.
MgSO4.X(H2O)-------> MgSO4 +X( H2O)
IF , n mole of hydrated mgso4 taken in the reactant side then , n mole of
anhydrous MgSO4 produce according to reaction.
n= 1. 547 /18x +120;
which is equal to mole of anhydrous MgSO4 that is
n= 0. 7554 /120;
by equating both equations
we get ,
0. 7554 /120 = 1. 547 /18x +120;
x= 7 (nearly.)
hence, 7 waters of hydration were attached to the MgSO4.
learn more about anhydrous MgSO4.--
https://brainly.com/question/28282924
#SPJ4
How many moles of H₂O can be produced when 5.5 moles of CO₂ is produced? C₂H₄ + 3 O₂ --> 2 CO₂ + 2 H₂O
Answers
Answer:
5.5 mol H2O
Explanation:
Given over what you need and then mole ratio of coefficients in the balanced equation then cross multiply and do PEMDAS for that, and then you got your answer :) hope this helped.

In metals, reactivity increases ____ a group, and in non-metals reactivity increases _____ a group.
A. Up, down
B. Up, up
C. Down, down
D. Down, up
Answers
Periodic table is divided into three metals, non metals and metalloids. The non metals are kept on the right side of the periodic table. Th correct option is option D.
What are non metals?
Non metals are the element that have property to gain electron. When any element gain electron then element attain negative charge and that element is called anion. The examples of non metals are Oxygen, nitrogen, carbon, Fluorine etc.
The properties of non metals are
Non metals are soft.
Non metals are not malleable that is they can be broken into thin sheets.
Non metals are not ductile they can not be broken into thin wires.
Non metals are brittle in nature that is they can be broken down easily.
Non metals are not lustrous.
In metals, reactivity increases down a group, and in non-metals reactivity increases up a group.
Therefore the correct option is option D.
To learn more about non metals, here:
https://brainly.com/question/28650063
#SPJ1
Answer:
d
Explanation:
same question
The table shows the charge on three unknown subatomic particles.
X positive
Y no charge
Z negative
Which particle is most likely present in the space outside of the nucleus of the atom?
Answers
Answer:
Z negative
Explanation:
The negative charge shows that this is an electron. Electrons are present outside the nucleus of the atom and having negative charge. Usually represented by e-
Answer:
Only Z?
Explanation:
what are some things you may see witness when a chemical reaction takes place
Answers
which class of amines can form intermolecular hydrogen bonds?
Answers
Primary and secondary amines can form intermolecular hydrogen bonds.
How do primary and secondary amines participate in intermolecular hydrogen bonding?Primary and secondary amines, which are a class of organic compounds, can participate in intermolecular hydrogen bonding. Intermolecular hydrogen bonding occurs when the hydrogen atom attached to the nitrogen atom in the amine molecule forms a hydrogen bond with another electronegative atom, such as oxygen or nitrogen, in a neighboring molecule.
Intermolecular hydrogen bonding is a type of attractive force between molecules and plays a crucial role in various chemical and physical properties. In the case of primary and secondary amines, the presence of a hydrogen atom bonded directly to the nitrogen atom allows for the formation of hydrogen bonds with other molecules. These hydrogen bonds enhance the intermolecular forces between the amines, leading to higher boiling points and increased solubility in polar solvents.
The ability of primary and secondary amines to form intermolecular hydrogen bonds is significant in biological systems and organic chemistry reactions. It influences molecular interactions, stability, and the behavior of compounds containing amine functional groups.
Learn more about hydrogen bonding
brainly.com/question/31139478
#SPJ11
The mass number of a chromium atom is 52 and it has 24 protons. How many neutrons does this atom have? 24 28 76 80
Answers
The number of protons and neutrons together makes the total atomic mass of the element. The atom of chromium has 28 neutrons. Thus, option b is correct.
What is an atomic mass?An atomic mass is the property of an element that defines the number of protons and neutrons of the atom placed in a periodic table. The atomic mass is represented at the lower half of the atomic symbol.
The atomic mass is the sum of the neutrons and the protons that are held together in the nucleus of the atom as a concentrated mass. The atomic number is given as,
Atomic number = number of protons + number of neutrons
Given,
The atomic mass of chromium = 52
Number of protons = 24
Substituting values above:
52 = 24 + number of neutrons
number of neutrons = 52-24
= 28
Therefore, the number of neutrons of a chromium atom is 28.
Learn more about atomic mass here:
https://brainly.com/question/17067547
#SPJ6
Answer: option b, 28
Which has the strongest intermolecular forces?
A B C or D
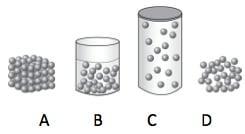
Answers
Answer:
A
Explanation:
PLZZ I WILL GIVE Braliest!!!
What makes a radioactive decay chain end?
A. The final element has the longest half-life.
B. All mass has been converted to energy.
C. The radiation is absorbed by something.
D. The final product is not radioactive.
SUBMIT
Answers
\(\huge{\mathbb{\tt {QUESTION:}}}\)
What makes a radioactive decay chain end?
OA. The final element has the longest half-life.
OB. All mass has been converted to energy.
OC. The radiation is absorbed by something.
OD. The final product is not radioactive.
\(\huge{\mathbb{\tt {ANSWER:}}}\)
D. The final product is not radioactive
\(\huge{\mathbb{\tt {EXPLANATION:}}}\)
A radioactive decay chain will end when the last particle formed is stable. The initial radioactive isotope is called the parent isotope.
#CarryOnLearning
#LetsEnjoyTheSummer
→X x K i m 0 2 x XA decay chain will end only when the final product is not radioactive.
What is radioactivity?The term radioactivity has to do with the sponteanous disintegration of an atom with the emission of radiation. Usually, there are radioactive decay series for different isotopes.
A radioactive decay series will continue untill a stable isotope is formed hence, a decay chain will end only when the final product is not radioactive.
Learn more about radioactivity: https://brainly.com/question/14356798
The list below includes some of the properties of butane, a common fuel. Identify the chemical properties in the list. Check all of the boxes that apply.
denser than water
burns readily in air
boiling point of –1.1°C
odorless
does not react with water
Answers
Answer:
Answer above is incorrect
Explanation:
The true answers are B and E on Edge 2020
Hope this helps :)
Answer:
B and E
Explanation:
on edge hope this helps
calculate the quantity of electerical charge needed to plate 1.386 mol cr from an acidic solution of
Answers
Question incomplete. Kindly provide all the information necessary to proceed further with the steps.
#SPJ11
what is the osmotic pressure (at 25°c) of seawater? it contains approximately 35.0 grams of nacl per liter. (seawater contains other stuff, but we'll ignore it.)
Answers
Therefore, the osmotic pressure of seawater, which contains approximately 35.0 grams of NaCl per liter, at 25°C is approximately 1498.59 Pa.
how to calculate the osmotic pressure ?
The osmotic pressure of seawater can be calculated using the Van't Hoff equation, which relates the osmotic pressure of a solution to its concentration and the temperature. The equation is given by:
Π = MRT
where M is the molarity of the solute, R is the ideal gas constant, and T is the temperature in kelvins. The molar mass of NaCl is 58.44 g/mol, so 35 g of NaCl in a liter of water corresponds to a molarity of:
M = 35 g / 58.44 g/mol = 0.599 mol/L
Converting the temperature from degrees Celsius to kelvins gives:
T = 25°C + 273.15 = 298.15 K
Substituting the values into the Van't Hoff equation gives:
Π = 0.599 mol/L * 8.31 J/(mol*K) * 298.15 K
Π = 1498.59 Pa
Therefore, the osmotic pressure of seawater, which contains approximately 35.0 grams of NaCl per liter, at 25°C is approximately 1498.59 Pa.
To learn more about osmotic pressure follow the given link: https://brainly.com/question/29714361
#SPJ1
What are the free radicals?
What are the negative health consequences of high amount of free radical in the system? (3 pts)
What are the antioxidants? (1.5 pts)
Answers
Free radicals are highly reactive molecules or atoms that have unpaired electrons in their outer shells.
Negative health consequences of a high amount of free radicals in the system include, Oxidative Stress, Inflammation, Cellular Damage.
Antioxidants are substances that can neutralize or counteract the damaging effects of free radicals.
Free radicals are highly reactive molecules or atoms that have unpaired electrons in their outer shells. They are formed as natural byproducts of various biological processes in the body, such as metabolism, immune response, and environmental factors like pollution, radiation, or smoking. Free radicals are unstable and seek to stabilize themselves by oxidizing other molecules in the body, leading to a chain reaction of damage to cells, proteins, and DNA.
Negative health consequences of a high amount of free radicals in the system include:
Oxidative Stress: Excessive free radicals can cause oxidative stress, which is an imbalance between the production of free radicals and the body's antioxidant defenses. This can result in damage to cellular components and contribute to the development of chronic diseases, including cancer, cardiovascular diseases, neurodegenerative disorders, and aging.
Inflammation: Free radicals can trigger and perpetuate inflammation in the body. Chronic inflammation is associated with various health conditions, including arthritis, asthma, diabetes, and autoimmune disorders.
Cellular Damage: Free radicals can damage cell membranes, proteins, and DNA, leading to mutations, cell dysfunction, and impaired cellular processes. This can disrupt normal cell function and contribute to the development of diseases.
Antioxidants are substances that can neutralize or counteract the damaging effects of free radicals. They help inhibit or reduce the oxidation of other molecules by donating an electron to stabilize the free radicals without becoming free radicals themselves. Antioxidants can be naturally occurring compounds found in fruits, vegetables, whole grains, nuts, and seeds, as well as synthetic substances. Some common antioxidants include vitamins C and E, beta-carotene, selenium, and various phytochemicals. Consuming a diet rich in antioxidants or supplementing with antioxidants can help protect against oxidative stress and mitigate the negative health consequences associated with high levels of free radicals.
To know more about Free radicals, click here, https://brainly.com/question/30045386
#SPJ11
Consider the reaction: ___ Al + ___ O2 --> ___ Al2O3. When 81 g of Al are reacted with 64 g of O2, _____ is the excess reactant and ______ of this reactant remain after the reaction is complete.
Answers
Answer:
Al is the excess reactant and 0.33 moles of this reactant remain after the reaction is complete
Explanation:
First of all, let's balance the equation:
4Al + 3O₂ → 2Al₂O₃
We need to know the moles of each reactant, to work with the stoichiometry.
Moles of Al: 81 g / 26.98 g/mol = 3 moles
Moles of O₂: 64 g / 32g/mol = 2 moles
If 4 moles of Al react to 3 moles of oxygen
3 moles of Al, may react with (3 . 3) / 4 = 2.25 moles of O₂
As we have 2 moles of oxygen and we need 2.25 moles, we do not have enough moles, so the O₂ is the limiting reactant. Then, the Al is the excess reactant.
3 moles of O₂ react to 4 moles of Al
Then, 2 moles of O₂ may react to (2 .4) / 3 = 2.67 moles of Al
We have 3 moles, and we need 2.67 moles, so there are 0.33 moles that still remain after the reaction goes complete (3 moles, we have - 2.67 moles we need)
Citric acid. C_6H_8O_7, is a triprotic acid found in a variety of fruits (e.g., lemons and limes). It, like acids in general and the unknowns in this lab, has a quite sour taste. (a) Write the balanced chemical equation describing the neutralization of citric acid with NaOH. (b) What volume of 0.3244 M NaOH is needed to completely neutralize 0.266 g of citric acid?
Answers
a) The balanced chemical equation describing the neutralization of citric acid (C₆H₈O₇) with NaOH is:
3NaOH + C₆H₈O₇ → Na₃C₆H₅O₇ + 3H₂O
b) To calculate the volume of 0.3244 M NaOH needed to neutralize 0.266 g of citric acid, we need to consider the stoichiometry of the balanced equation.
Determine the molar mass of citric acid:
C₆H₈O₇ has a molar mass of 192.12 g/mol.
Calculate the moles of citric acid:
Moles of citric acid = mass of citric acid / molar mass of citric acid.
Use the stoichiometry of the balanced equation to determine the moles of NaOH needed:
According to the balanced equation, 3 moles of NaOH react with 1 mole of citric acid.
Calculate the volume of 0.3244 M NaOH:
Volume of NaOH = (moles of NaOH needed / molarity of NaOH) * 1000.
Substituting the values and performing the calculations, you can determine the volume of 0.3244 M NaOH needed to completely neutralize 0.266 g of citric acid.
To know more about neutralization, refer here:
https://brainly.com/question/14156911#
#SPJ4
Why is the equilibrium constant for the KHT dissociating in water equal to the square of the bitartarate concentration
Answers
The equilibrium constant for the dissociation of potassium hydrogen tartrate (KHT) in water is equal to the square of the bitartrate concentration due to the stoichiometry of the dissociation reaction.
When KHT (potassium hydrogen tartrate) dissolves in water, it undergoes dissociation according to the following equilibrium reaction:
KHT ⇌ K⁺+ HT⁻
The equilibrium constant for this reaction, denoted as K, is defined as the ratio of the concentrations of the products ( K⁺ and HT⁻) to the concentration of the reactant (KHT).
K = [ K⁺] [HT⁻] / [KHT]
Now, let's consider the dissociation of HT⁻ (hydrogen tartrate) ion further:
HT⁻ ⇌ H⁺ + T²⁻
In this reaction, HT⁻dissociates into a hydrogen ion (H^+) and a bitartrate ion (T²⁻). Since KHT dissociates to produce one HT⁻ ion, we can say that the concentration of HT⁻ is equal to the concentration of KHT.
Therefore, [HT⁻] = [KHT]
Now, substituting this expression into the equilibrium constant equation:
K = [K⁺][HT⁻] / [KHT]
K = [K⁺][KHT] / [KHT]
K = [K⁺]
Hence, the equilibrium constant, K, for the dissociation of KHT in water is equal to the concentration of K⁺ ions.
Now, since HT⁻ is equal to KHT in concentration, we can rewrite the equilibrium constant equation as:
K = [K⁺][HT⁻]
K = [K⁺][KHT]
The concentration of bitartrate ions (T²⁻) is equal to half the concentration of HT⁻ ions (KHT), due to stoichiometry:
[T²⁻] = 0.5[HT⁻]
K⁺+ HT⁻ = 0.5[KHT]
Substituting this expression into the equilibrium constant equation:
K = [K⁺][KHT]
K = [K⁺](0.5[KHT])
K= 0.5[K⁺][KHT]
From this equation, we can see that the equilibrium constant K is equal to the square of the bitartrate concentration ([T²⁻]).
The equilibrium constant for the dissociation of KHT in water is equal to the square of the bitartrate concentration because the stoichiometry of the dissociation reaction shows that the concentration of bitartrate ions is equal to half the concentration of HT^- ions, and the equilibrium constant is proportional to the product of the concentrations of the ions involved in the reaction.
To know more about stoichiometry visit:
https://brainly.com/question/28780091
#SPJ11
The equilibrium constant for the KHT dissociating in water is equal to the square of the bitartarate concentration because of the stoichiometry of the reaction.In the reaction of KHT dissociating in water, KHT donates one hydrogen ion (H+) to water to form tartrate ion (T2-) and hydronium ion (H3O+).
This reaction is represented as:KHT(aq) + H2O(l) ⇌ T2-(aq) + H3O+(aq)This reaction has one mole of KHT reacting with one mole of water to produce one mole of tartrate ion and one mole of hydronium ion. At equilibrium, the concentration of KHT, water, tartrate ion, and hydronium ion will be constant. Let the initial concentration of KHT be ‘x’.
After reacting with water, let the concentration of KHT be ‘x – y’ and the concentration of tartrate ion and hydronium ion be ‘y’.
Therefore, the equilibrium expression for this reaction can be written as:KHT(aq) + H2O(l) ⇌ T2-(aq) + H3O+(aq)[T2-(aq)][H3O+(aq)]/[KHT(aq)][H2O(l)]Now, the concentration of water is assumed to be a constant since it is in large excess with respect to KHT.
Therefore, the equilibrium expression can be written as:[T2-(aq)][H3O+(aq)]/[KHT(aq)]The square of the bitartarate concentration is used in this expression because two moles of bitartarate are obtained from one mole of KHT.
Hence, the equilibrium constant (Kc) expression can be written as:Kc = [T2-(aq)][H3O+(aq)]/[KHT(aq)] = [y]2/[(x – y)]where,‘x’ is the initial concentration of KHT, and‘y’ is the amount of KHT that has reacted to form tartrate ion.
Therefore, the equilibrium constant for the KHT dissociating in water is equal to the square of the bitartarate concentration.
To know more about equilibrium constant refer here : brainly.com/question/32691630
#SPJ11
suppose you misread the final volume of base in the buret and record a volume that is greater than the true value. will your final calculated percent of acetic acid in vinegar be too high, too low, or unaffected?
Answers
The buret's final base volume and record a volume that is higher than the actual value. Acetic acid content in vinegar will be overestimated in the final calculation.
Explain about the Acetic acid?In addition to its other names, acetic acid is also referred to as ethanoic acid, ethylic acid, vinegar acid, and methane carboxylic acid; its chemical formula is CH3COOH. Vinegar gets its distinctive smell from acetic acid, a byproduct of fermentation. A 4-6% acetic acid solution makes up vinegar.
Vinegar, which includes 4 to 18% acetic acid, has acetic acid as its primary ingredient. It serves as an ingredient and food preservative (known as E260).
Acetic acid is a strong irritant to the eyes, mucous membranes, upper respiratory tract, and skin when it is in vapor form. 80% or higher acetic acid solutions can be corrosive when they come into contact with the skin or eyes, severely burning any exposed tissue.
To learn more about Acetic acid refer to:
https://brainly.com/question/24304533
#SPJ4
A car engine fueled by gasoline uses which kind of energy?
OA. Electric
OB. Nuclear
O C. Potential
OD. Chemical
Answers
Answer:
A.electric
Explanation:
electric is very useful in ingines
Answer: A.
Explanation:
In a metal lattice, the metal ions are _____ and they are _____.
Answers
Answer:
a.)cations; free to move around
b.)cations; fixed in place
Which of the following statements is incorrect?
O Free radicals are dangerous because they emit energy.
O isotopes have the same atomic number but different atomic mass.
O atoms have about the same numbers of protons and electrons.
O All molecules are made of atoms.
Answers
Answer: Free radicals are dangerous because they emit energy is incorrect.
Explanation:
Free radicals are dangerous because they are highly reactive species with an unpaired electron in their outer shell. This unpaired electron can react with other molecules, including DNA, proteins, and lipids, which can lead to damage and disease. However, free radicals do not emit energy as a general rule.
Isotopes having the same atomic number but different atomic mass is a correct statement. Atoms having the same number of protons and electrons is also a correct statement since atoms are electrically neutral. Finally, all molecules are indeed made of atoms.