specify the degree of unsaturation (index of hydrogen deficiency) of the following formulas: (a) c24h24 13 (b) c7h6brcl 4 (c) c9h11n submit answer
Answers
The degree of unsaturation for the given formulas is as follows:
(a) C₂₄H₂₄: 36
(b) C₇H₆BrCl: 12
(c) C₉H₁₁N: 12.5
To determine the degree of unsaturation (index of hydrogen deficiency) in a formula, we can use the formula:
Degree of unsaturation = \(\[(2n + 2) - \frac{h + x}{2}\]\)
where n is the number of carbon atoms, h is the number of hydrogen atoms, and x is the number of halogen atoms (if present).
(a) C₂₄H₂₄:
Degree of unsaturation = \(\[(2 \times 24 + 2) - \frac{24 + 0}{2}\]\)
= 48 - 12
= 36
The degree of unsaturation for C₂₄H₂₄ is 36.
(b) C₇H₆BrCl:
Degree of unsaturation = \(\[(2 \times 7 + 2) - \frac{6 + 1 + 1}{2}\]\)
= 14 - 2
= 12
The degree of unsaturation for C₇H₆BrCl is 12.
(c) C₉H₁₁N:
Degree of unsaturation = \(\[(2 * 9 + 2) - \frac{11 + 0}{2}\]\)
= 18 - 5.5
= 12.5
The degree of unsaturation for C₉H₁₁N is 12.5.
To know more about the degree of unsaturation refer here :
https://brainly.com/question/31745187#
#SPJ11
Related Questions
What is the relationship between the number of carbon atoms in hydrocarbon at its boiling point ?
Please answer correctly with explanation.
Will give the brainliest !!
Answers
So
\(\\ \tt\longmapsto C\propto BP\)
No of carbon atoms is directly proportional to the boiling point of the compound.If no of carbon atoms increases in a hydrocarbon the length of carbon chain also increases and all carbons get wrapped around themselves.Which causes increase in boiling point of compound.Look the table below for the boiling points of some alkanes
\(\boxed{\begin{array}{c|c}\boxed{\bf Alkane}&\boxed{\bf Boiling point}\\ \sf CH_4&\sf -164°C \\ \sf C_2H_6 &\sf -88.6°C \\ \sf C_3H_8&\sf 42.1°C \\ \sf C_4H_{10} &\sf -0.5°C \\ \sf C_5H_{12} &\sf 36.1°C \\ \sf C_6H_{14} &\sf 68.9°C \\ \sf C_7H_{16} &\sf 98.4°C \\ \sf C_8H_{18} &\sf 125.7°C \\ \sf C_9H_{20} &\sf 150.4°C \\ \sf C_{10}H_{22} &\sf 150.7°C \end{array}}\)
How does peer review affect research?

Answers
Experimental results will be trusted by the scientific community only if they have been peer-reviewed. Thus, option A is correct.
What is research?Research is the creation of new knowledge and/or the use of existing knowledge in a new and creative way so as to generate new concepts and understandings.
“Peer-reviewed” means that the paper is analyzed by fellow scientists, who evaluate the logic or methodology that might shed doubt on the findings.
Learn more about the research here:
https://brainly.com/question/14389222
#SPJ2
mercury thermometer should not be placed in the mouth of the children why?
Answers
Answer: If a pinch of mercury is consumed you will die from it so using a mercury thermometer is very unsafe if its breaks and a child consumes that mercury.
Explanation:
Don't use one
If the glass breaks and the mercury is not properly cleaned up, the little silvery ball within a mercury thermometer might be hazardous. As the mercury evaporates, it may pollute the air around and turn dangerous to both people and animals.
Children that have been exposed to mercury have lower IQs, hearing impairments, and worse coordination.
Long-term exposure worsens and exacerbates symptoms, which may lead to personality changes or even coma.
Mercury thermometers can be replaced by a number of things:
electronic thermometersGlass thermometers with gallium tinalcohol thermometers in glassThese non-mercury fever thermometers are significantly safer and equally accurate as mercury thermometers.
Read more about Mercury Thermometers :
https://brainly.com/question/27323100
1. What temperature is equivalent to 32°F?
Answers
Answer:
0 Celsius
Explanation:
its 0 degrees C
Answer:
0°C = 32°F
Explanation:
Neptune _____.
is a terrestrial planet
has a nearly horizontal axis
was discovered by mathematical calculations
is the largest planet in the solar system
Answers
Answer:
C. Was Discovered by Mathematical calculations
Explanation:
Terrestrial Planets are Inner Planets (Mercury,Venus,Earth,Mars) and Neptune is an ice giant or gas giant, Neptune is also not the largest planet in the solar system, I believe that's Jupiter. I don't know for sure if Neptune has a horizontal axis but my best guess is C. I hope this helps! :^)
If 3.22 g of precipitate are recovered from the reaction of limewater (Ca(OH),) with carbon dioxide to produce
water and calcium carbonate, what is the percent yield if 20.0 g of carbon dioxide was mixed with the
limewater?
the correct answer is 7.1% but i don't understand what they did
Answers
Answer:
Percent yield = 7.1%
Explanation:
The general reaction of the problem is:
Ca(OH)₂ + CO₂ → H₂O + CaCO₃
To solve this question we need to find theoretical yield using the amount of carbon dioxide added because:
Percent yield = Actual yield (3.22g) / Theoretical yield * 100
Theoretical yield is the maximum amount of product that could be obtained. To find it we need to convert the mass of CO₂ to moles. The moles of CO₂ = Moles of CaCO₃:
Moles CO₂ -Molar mass: 44.01g/mol):
20.0g * (1mol / 44.01g) = 0.454 moles CO₂ = Moles of CaCO₃ produced
Mass CaCO₃ = Theoretical yield -Molar mass: 100.09g/mol-:
0.454 moles * (100.09g / mol) = 45.5g of CaCO₃ = Theoretical yield.
Replacing:
Percent yield = 3.22g / 45.5g * 100
Percent yield = 7.1%a certain metal crystallizes in a face-centered cubic arrangement and has a density of a density and atomic radius of 6.657 g/cm3 and 183.5 pm, respectively. which element below is it?
Answers
The element that crystallizes in a face-centered cubic (FCC) arrangement with a density of 6.657 g/cm³ and an atomic radius of 183.5 pm is Aluminum (Al).
The formula for calculating the density (ρ) of a metal with a face-centered cubic structure is:ρ = (Z × M) / (a³ × N_A),where Z is the number of atoms in a unit cell, M is the molar mass of the metal, a is the edge length of the unit cell, and N_A is Avogadro's number (6.022 × 10²³ mol⁻¹).
In the case of a face-centered cubic structure, Z is 4, and a is related to the atomic radius (r) as follows:
a = 4√(2) × r. Given that the density (ρ) is 6.657 g/cm³ and the atomic radius (r) is 183.5 pm (or 1.835 Å), we can substitute these values into the equations and solve for the molar mass (M).
r = 183.5 pm = 183.5 × 10⁽⁻²³⁾ cm.
Next, calculate the edge length (a) using the atomic radius:
a = 4√(2) × r = 4√(2) × 183.5 × 10⁽⁻¹⁰⁾ cm.
M = (ρ × a³ × N_A) / (Z).
M = (6.657 g/cm³ × (4√(2) × 183.5 × 10^(-10) cm)³ × 6.022 × 10²³mol⁽⁻¹⁾) / 4.
The element that crystallizes in a face-centered cubic (FCC) arrangement with a density of 6.657 g/cm³ and an atomic radius of 183.5 pm is Aluminum (Al).
Learn More About Face-Centered Cubic Arrangement
brainly.com/question/4501234
#SPJ4
Can you tell me the density of a sample of ozone gas O3 at 755 Torr and 18C
Answers
124g/ml is the density of a sample of ozone gas at 755 Torr and 18C. Density is the mass of an object substance per unit volume.
Density is the mass of an object substance per unit volume. d = M/V, wherein d is density, M is the weight, and V is volume, is the formula for density. Grammes per cubic centimetre are a typical unit of measurement for density.
For instance, while Earth has a density of 5.51 grammes per cubic centimetre, water has a density of 1 grammes per cubic centimetre. The metre-kilogram-second (or SI) unit for density is kilogrammes per cubic metre. For instance, air weighs 1.2 kilogrammes per cubic metre.
Density=PM/RT
= 755 ×48/0.0821×291
=124g/ml
To know more about density, here:
https://brainly.com/question/29775886
#SPJ1
name the process Wich take place when .a solid carbon lv dry ice changes directly into gas
Answers
Answer:
evaporation takes place
Can you help plzzz thank you
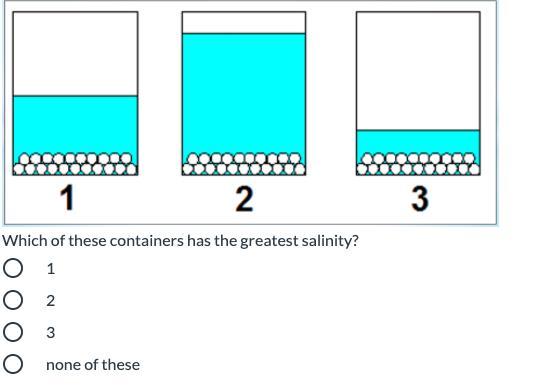
Answers
Answer:
It would be Container 3
Explanation:
Each of the containers has the same amount of salt. Salinity refers to salt level. Since the question is asking for the container with the greatest salinity, you are looking for the container with the least water (because it will be the saltiest out of all of them). Container 3 has the least water.
Hope this helps :)
3 has less water so its saltier
The specific heat capacity of zinc 0.386 J/g °C. How many joules would be released when 156 grams of zinc at 28.0°C were heated to 96.0 °C?
Answers
Answer:
The following data were obtained from the question:
Specific heat capacity (C) = 0.386 J/gºC
Mass (m) = 156 g
Initial temperature (T1) = 96.0ºC
Final temperature (T2) = 28.0ºC
Change in temperature (ΔT) = T1 – T2 = 96 – 28 = 68°C
Heat (Q) =..?
The heat released can be obtained as follow:
Q = m * c * ΔT
= 156 g * 0.386 J/g °C * 68.0 °C
≈ Q = m * c * ΔT
= 156 g * 0.386 J/g °C * 68.0 °C
≈ 3977.568 J
Therefore, the heat released is 3977.568 J
Explanation:
dimethylhydrazine, (ch3)2nnh2, was used as a fuel for the apollo lunar descent module, with n2o4 being used as the oxidant. the products of the reaction are h2o, n2, and co2.
Answers
Dimethylhydrazine (CH₃)₂NNH₂ is a fuel used for the Apollo Lunar Descent Module, and N₂O₄ is the oxidant. During the reaction, H₂O, N₂, and CO₂ are produced.
The fuel and oxidizer were stored separately to prevent any accidental reaction before use. Once the engines were ignited, the dimethylhydrazine and N₂O₄ mixed and reacted, producing energy and the products mentioned above.
The fuel used in Apollo Lunar Descent Module was dimethylhydrazine (CH₃)₂NNH₂, whereas the oxidant was N₂O₄. The two substances were stored separately, and the reaction was initiated only when they were mixed. The reaction between the fuel and oxidant produced H₂O, N₂, and CO₂.
The reaction between dimethylhydrazine and nitrogen tetroxide was exothermic and highly energetic.The reason why dimethylhydrazine was chosen as the fuel is that it is highly flammable and can quickly produce large amounts of heat and energy when mixed with an oxidant.
This makes it an ideal fuel for rocket engines, especially those used in space exploration, where high-performance and efficiency are critical.
The reaction between dimethylhydrazine and nitrogen tetroxide is also highly stable and controllable, making it suitable for use in the Apollo Lunar Descent Module.
To know more about exothermic click on below link:
https://brainly.com/question/4345448#
#SPJ11
Which of the following pairs of elements will combine to produce an ionic
bond?
A.Lithium and Bromine
B.Oxygen and Hydrogen
C.Carbon and Oxygen
D.Nitrogen and Fluorine
Answers
Answer:
A. Lithium and Bromine (LiBr)
Explanation:
Ionic bonds are chemical compounds formed between a metal and nonmetal. Lithium is a metal and Bromine is a nonmetal, therefore they would combine to produce an ionic bond.
- Oxygen and Hydrogen are both nonmetals, therefore they cannot produce an ionic bond when combined.
- Carbon and Oxygen are both nonmetals, therefore they cannot produce an ionic bond when combined.
- Nitrogen and Fluorine are both nonmetals, therefore they cannot produce an ionic bond when combined.
2 Points
What charge would an ion have if it had more electrons than protons?
A. It would be neutral, since electrons would be outside the nucleus.
B. It would have no charge.
C. It would have a net negative charge.
D. It would have a net positive charge.
SUBMIT
Answers
Answer: C, negative
Explanation: Electrons give a negative charge, so having more electrons then postiivley charged protons would result in a negative charge.
given that the mean relating atomic mass of chlorine gas is 35.5 and contains a mixture of two isotopes of mass number 35 and 37 . Calculate the ratio of each isotope in the naturally occurring element
Answers
The abundance of isotope 35 = 75%
The abundance of isotope 37 = 25%
Ratio isotope 35 : isotope 37 = 3 : 1
Further explanationGiven
Ar Cl = 35.5
Two isotopes of mass number 35 and 37
Required
The ratio of each isotope
Solution
If the abundance of isotope 35 is x%, then the abundance of isotope 37 is (100-x)%
35.5 = x%.35 + (100-x)%.37
35.5 = 0.35x+0.37(100-x)
35.5=0.35x-0.37x+37
0.02x=1.5
x=75
what is the h+ oh- and poh of solution with ph=3.67
Answers
A brine solution of salt flows at a constant rate o f7 L/min into a large tank that initially held100 L of brine solution in which was dissolved 0.25kg of salt. The solution inside the tank is kept well stirred and flows out of the tank at the same rate. If the concentration of salt in the brine entering the tank is 0.05kg/L, determine the mass of salt in the tank after t min. When will the concentration of salt in the tank reach 0.03kg/L?
Answers
The mass of salt in the tank after t minutes is 0.25 kg.
The concentration of salt in the tank will reach 0.03 kg/L after 4 minutes.
The first step requires us to determine the mass of salt in the tank after a certain time, t minutes. Since the brine solution flows at a constant rate of 7 L/min into the tank and the concentration of salt in the brine entering the tank is 0.05 kg/L, the rate of salt inflow is 7 L/min × 0.05 kg/L = 0.35 kg/min.
However, the rate of outflow is also 7 L/min, resulting in an equal rate of salt outflow. Therefore, the net change in the mass of salt over time is zero. Consequently, regardless of the duration, the mass of salt in the tank remains constant at 0.25 kg.
Moving on to the second step, we need to find out when the concentration of salt in the tank will reach 0.03 kg/L. Since the inflow and outflow rates remain constant, the concentration of salt in the tank will decrease linearly over time.
Starting with an initial concentration of 0.05 kg/L and considering the equal rates of inflow and outflow, it takes 2 minutes for the concentration to decrease to half, i.e., 0.025 kg/L. Similarly, it will take an additional 2 minutes for the concentration to decrease from 0.025 kg/L to 0.03 kg/L. Therefore, the concentration of salt in the tank will reach 0.03 kg/L after 4 minutes.
Learn more about concentration
brainly.com/question/30862855
#SPJ11
"pure water does not conduct electricity. But some tap water probably can. Which best explains this?" a . Tap water is H20 b . Pure water is H20 c . Some tap water has minerals, such as iron in it.
Answers
Explanation:
"pure water does not conduct electricity. But some tap water probably can."
The reason why pure water does not conduct electricity is due to the absence of mineral and metal containing salts. Pure water is made up of only Hydrogen and oxygen atom as represented by the formula; H2O
Tap water on the other hand are most likely able to conduct electricity due to the presence of these ions. They may arise from impurities or during treatment processes.
The correct options are;
b . Pure water is H2O
c . Some tap water has minerals, such as iron in it.
An old refrigerator is rated at 500 W how many kilowatt hours of electric energy what does refrigerator use in 30 days assume the refrigerator is running 12 hours per day
Answers
The refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
To calculate the kilowatt-hours (kWh) of electric energy used by the refrigerator in 30 days, we need to multiply the power rating by the total running time.
Given:
Power rating of the refrigerator = 500 W
Running time per day = 12 hours
Number of days = 30
First, we need to convert the power rating from watts to kilowatts:
Power rating = 500 W / 1000 = 0.5 kW
Next, we calculate the total energy used in kilowatt-hours (kWh) over the 30-day period:
Energy used = Power rating × Running time × Number of days
Energy used = 0.5 kW × 12 hours/day × 30 days
Energy used = 180 kWh
Therefore, the refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
For more question on energy
https://brainly.com/question/29339318
#SPJ8
Aluminum reacts with excess hydrochloric acid to form aqueous aluminum chloride and 35.8 mL of hydrogen gas over water at 27 degrees C and 751mmHg. How many grams of aluminum reacted?
Answers
The reaction of aluminum with excess hydrochloric acid produced 35.8 mL of hydrogen gas at 27°C and 751mmHg, hence, 1.74 grams of aluminum reacted.
The balanced chemical equation for the reaction between aluminum and hydrochloric acid can be expressed as:
2Al + 6HCl → 2AlCl₃ + 3H₂
From the equation, it is evident that two moles of aluminum react with six moles of HCl to produce two moles of aluminum chloride and three moles of hydrogen gas. We can use the ideal gas law to calculate the number of moles of H₂ gas that were produced, as follows:
PV = nRT
n = PV/RT
n(H₂) = (751/760)(35.8/1000)/(0.0821)(300) = 0.00145 moles
Since the molar ratio of Al to H₂ is 2:3, we can calculate the number of moles of Al that reacted:
n(Al) = (2/3)n(H₂) = (2/3)(0.00145) = 0.0009667 moles
Finally, we can use the molar mass of Al to calculate the mass of Al that reacted:
m(Al) = n(Al) × M(Al) = 0.0009667 × 26.98 = 0.0261 g ≈ 1.74 g
Therefore, 1.74 grams of aluminum reacted.
Learn more about moles here:
https://brainly.com/question/15209553
#SPJ11
complete the fission reaction.235U + 1 neutron → [X] +141Ba + 3 neutronsMass Number:Chemical Symbol:
Answers
It's important to know that the reaction must have the total mass involved before and after due to the law of conservation of mass. This means that the total mass number of the left side of the reaction must be equal to the right side. So, we have the following
\(235+1=X+141+3\)Notice that 235 is the mass number of U, 1 is the mass number of the neutron, X represents the mass number of the unknown element, 141 represents the mass number of Ba, and 3 represents the mass number of three neutrons. This means X should have a mass number of 92, which is the element Kr.
Therefore, the mass number is 92 and the chemical symbol is Kr.
Question 1 (1 point) 1. Ca Cl 4. S-Se 7. Br-1 2.0-H 5. C-Cl 8. Na-Bri 3. N-Bri 6. P-I 9. Cl-Cl Four of the bonds in the table above can be classified as polar covalent. Which numbers correspond to the
Answers
The bonds that can be classified as polar covalent are 1 (Ca-Cl), 4 (S-Se), 5 (C-Cl), and 9 (Cl-Cl). These bonds involve elements with different electronegativities, resulting in an unequal sharing of electrons and a partial positive and partial negative charge distribution.
Start by identifying the bonded atoms for each pair of elements in the table. The pairs of elements are as follows:
C-Cl
Na-Br
N-Br
S-Se
C-Cl
P-I
Br-1
Na-Br
Cl-Cl
Look up the electronegativity values for each element. Electronegativity is a measure of an atom's ability to attract electrons in a bond. The greater the electronegativity difference between two atoms, the more polar the bond.
Here are the electronegativity values for the elements involved in the pairs:
C: 2.5
Cl: 3.0
Na: 0.9
Br: 2.8
N: 3.0
S: 2.5
Se: 2.6
P: 2.2
I: 2.5
Calculate the electronegativity difference for each pair of elements by subtracting the electronegativity value of the less electronegative atom from the value of the more electronegative atom.
Here are the electronegativity differences for each pair:
C-Cl: 3.0 - 2.5 = 0.5
Na-Br: 2.8 - 0.9 = 1.9
N-Br: 2.8 - 3.0 = -0.2 (not a polar covalent bond)
S-Se: 2.6 - 2.5 = 0.1
C-Cl: 3.0 - 2.5 = 0.5
P-I: 2.5 - 2.2 = 0.3
Br-1: 2.8 - 1.0 = 1.8 (not a polar covalent bond)
Na-Br: 2.8 - 0.9 = 1.9
Cl-Cl: 3.0 - 3.0 = 0 (not a polar covalent bond)
Arrange the bonds in ascending order based on the electronegativity difference.
The bonds listed from least polar covalent to most polar covalent are:
C-Cl (Electronegativity difference = 0.5)
Na-Br (Electronegativity difference = 1.9)
S-Se (Electronegativity difference = 0.1)
P-I (Electronegativity difference = 0.3)
Therefore, the numbers corresponding to these polar covalent bonds in numerical order are: 5, 8, 4, 6.
Learn more about electronegativity here
https://brainly.com/question/29597673
#SPJ11
100 POINTS!!! ANSWER ASAP

Answers
Answer:
1eV
Explanation:
Answer:
1ev
Explanation:
A gas at constant volume has a pressure of 4. 50 atm at 200. K. What will be the pressure of the gas at 250. K? 3. 60 atm 4. 60 atm 5. 63 atm 5. 89 atm.
Answers
5.625 atm will be the pressure of the gas at 250 K temperature of the gas at constant volume.
How we calculate the pressure of the gas?Pressure of the gas will be calculated by using the ideal gas equation as:
PV = nRT,
From the question, it is clear that:
Moles of the gas and volume is constant here, so we calculate the pressure by rearranging the above equation as:
P/T = nR/V
And required equation will be:
P₁/T₁ = P₂/T₂, where
P₁ = pressure of gas = 4.50 atm
T₁ = temperature of gas = 200 K
P₂ = pressure of gas = to find?
T₂ = temperature of gas = 250 K
On putting all these values in the above equation, we get
P₂ = 4.50 × 250 / 200 = 5.625 atm
Hence, 5.625 atm is the pressure of the gas.
To know more about ideal gas equation, visit the below link:
https://brainly.com/question/1056445
identify the limiting and excess reagent when there are 40.0g of silicon and 25.5g of nitrogen
Answers
Answer:
Explanation:
To identify the limiting and excess reagents, we need to compare the moles of each reactant and determine which one is present in a stoichiometrically lesser amount.
Given:
Mass of silicon (Si) = 40.0 g
Mass of nitrogen (N2) = 25.5 g
To determine the moles of each reactant, we need to divide their masses by their respective molar masses.
Molar mass of silicon (Si) = 28.0855 g/mol
Molar mass of nitrogen (N2) = 28.0134 g/mol
Moles of silicon (Si) = 40.0 g / 28.0855 g/mol ≈ 1.424 mol
Moles of nitrogen (N2) = 25.5 g / 28.0134 g/mol ≈ 0.911 mol
Next, we need to determine the stoichiometric ratio of the reactants based on the balanced chemical equation. Let's assume the balanced equation is:
3Si + 2N2 → Si3N4
From the balanced equation, we can see that 3 moles of silicon react with 2 moles of nitrogen to form 1 mole of silicon nitride.
Comparing the mole ratio of silicon to nitrogen, we have:
1.424 mol Si : 0.911 mol N2
Since the ratio is not a whole number ratio, we can simplify it by dividing both sides by 0.911:
1.424 mol Si / 0.911 mol N2 ≈ 1.56
Based on this ratio, we can see that the stoichiometric ratio of silicon to nitrogen is approximately 1.56:1. This means that 1.56 moles of silicon react with 1 mole of nitrogen.
Now, comparing the given moles of silicon and nitrogen to the stoichiometric ratio, we can determine the limiting and excess reagents.
Since we have 1.424 mol of silicon and the stoichiometric ratio requires only 1.56 moles of silicon per mole of nitrogen, silicon is the limiting reagent.
Nitrogen, with 0.911 mol, is in excess because it is not fully consumed in the reaction.
In conclusion:
Silicon (Si) is the limiting reagent.
Nitrogen (N2) is the excess reagent.
Example A:
Julio was given the task to produce 59 grams of iron(III) oxide, . They had one tank of oxygen gas, and 54 grams of solid iron, . They knew that if he heated the iron in the presence of the oxygen gas, they could produce the iron(III) oxide. Since they had such little amount of iron, they decided to use as little of the iron as they could to produce the 59 grams of iron(III) oxide. Julio measured out 41 grams of iron and heated it with excess oxygen. Assume Julio's reaction went 100%. Julio was very happy that their reaction produced the 59 grams.
Example B:
Gretchen was given the task to produce 19 grams of aluminum fluoride, . They had a tank of fluorine gas, , and 7 grams of solid aluminum, . They knew that if they heated the aluminum in the presence of excess fluorine gas, they could produce the aluminum fluoride. Since Gretchen had such little amount of aluminum, they decided to use as little of aluminum as they could to produce the 19 grams of aluminum fluoride. Gretchen measured out 5 grams of aluminum and heated it with excess fluorine gas. Assume Gretchen's reaction went 100%. Gretchen was very happy their reaction produced the 19 grams.
Question #1: Which scientist did the experiment incorrectly? (1 pt)
Question #2: Explain what the scientist did incorrectly. Be sure to include the balanced equation for each scientist. Show the stoichiometry and explain in complete sentences why one experiment is wrong and the other is not. (4 pts)
Question #3: Explain specifically what the scientist should do to make the experiment correct (use numbers to support your answer). Also, include the stoichiometry to show how the scientist can make the experiment correct. (3 pts)
Answers
As Scientist B did the experiment incorrectly tht is Gretchen measured out 5 grams of aluminum and heated it with excess fluorine gas.
What is the stoichiometry in chemistry?Chemical Stoichiometry refers back to the quantitative look at of the reactants and merchandise concerned in a chemical reaction. The word “ stoichiometry” is derived from the Greek word “stoikhein” which means detail and “metron” which means measure.
As scientists used the insufficient amount of Al due to which weight of Al2O3 is not found ane caused the high error.It includes stoichiometry to show how the scientist can make the experiment correct by the production of 19 g of ALF3 which is 26.98 x 19 / 83.98 g of Al.so the mass is 610 g of Al that would be the correct amount.Read more about stoichiometry :
https://brainly.com/question/16060223
#SPJ1
Will give brainliest but hurry
The condensation point of steam is the same temperature as the ______ of water.
A) freezing point
B) melting point
C) boiling point
Answers
The capacity of an inflated balloon is 100 cm³.How many balloons can be filled with the gas from a 20L helium gas tank?
Answers
The gas from a 20L helium gas tank can fill approximately 200 balloons.
To determine the number of balloons that can be filled with the gas from a 20L helium gas tank, we need to calculate the volume of helium gas in the tank and then divide it by the capacity of each balloon.
Given:
Volume of helium gas tank = 20 L
Capacity of each balloon = 100 cm³
First, we need to convert the volume of the helium gas tank to cubic centimeters (cm³) since the capacity of the balloon is given in cm³.
20 L = 20,000 cm³
Now, we can divide the volume of the helium gas tank by the capacity of each balloon:
Number of balloons = (Volume of helium gas tank) / (Capacity of each balloon)
Number of balloons = 20,000 cm³ / 100 cm³ = 200
Therefore, the gas from a 20L helium gas tank can fill approximately 200 balloons.
for more such questions on helium gas
https://brainly.com/question/30331443
#SPJ8
write a balanced equation from the following cell notation. include the physical state of each reactant and product. al(s) al^3 (aq)
Answers
Net balanced equation is- 2Al(s) + 3Ni²⁺(aq) → 2Al³⁺(aq) + 3Ni(s)
In chemistry, a cell notation or cell representation is a simplified way to express an electrochemical cell reaction.
By omitting all other common ions and inactive substances, the two half-cells are represented in cell notation by writing the formula of each unique chemical species involved in the redox reaction across the cell. The species in each half-cell are grouped together and each species is divided by a vertical bar. A salt bridge is represented by two vertical bars or slashes separating the two half-cells (which generally contains an electrolyte solution such as possasium nitrate or sodium chloride that is left unwritten). It is customary to place the aqueous species closest to the double bar and to represent the anode to the left and the cathode to the right of the double bar.
Aluminium acts as anode while nickel act as a cathode.
Oxidation - Al(s) → Al³⁺(aq) + 3e⁻ x2
Reduction - Ni²⁺(aq) + 2e⁻ → Ni(s) x3
Thus, Net balanced equation- 2Al(s) + 3Ni²⁺(aq) → 2Al³⁺(aq) + 3Ni(s)
To learn more about cell notation refer- https://brainly.com/question/27029321
#SPJ4
Your question is incomplete please find the complete question over the internet.
How many grams of KF are in 2 liters of a 3.0 M solution of KF
Answers
Answer:
mass ( g ) = 348 g
Explanation:
First you know : M = mole / volume (L)
in question you have the M and V and the formula of SUBSTANCE ( KF )
first you get the number of mole from equation above
so 3 = no of mole / 2
no of mole = 3 × 2 = 6 moles
and the moles equation is no of moles = mass ( g ) / molecular weight ( g/mole )
so you have already calculate the moles and you can know the MW from the Question
Mw of KF = 39 + 19 = 58
so n = mass / MW
so 6 = mass / 58
mass ( g ) = 348 g
GOOD LUCK