Answers
Answer:
WHY DO YOU NEED HELP HUH
DON'T CHEAT READ AND STUDY
Explanation:
Answer:
It's C
Explanation:
Related Questions
Pls help ill mark you brainlist asap pls

Answers
Answer:
Add Copper(II) oxide, CuO, to an aqueous solution of hydrochloric acid, HCl, to produce the salt copper chloride, CuCl2. To obtain a pure, dry sample of the salt, remove excess, undissolved CuO through filtration. Then heat the system to evaporate water, leaving behind pure Copper(II) Oxide.
A chemist prepares a solution by dissolving 4.585 g of nano3 in enough water to make 200 ml of solution. what molar concentration of sodium nitrate should appear on the label? if the chemist mistakenly makes 225 ml of the solution instead of the 200 ml, what molar concentration of sodium nitrate will the chemist have actually prepared?
Answers
0.2695 M is molar concentration of sodium nitrate should appear on the label and if the chemist mistakenly makes 225 ml of the solution instead of the 200 ml, 0.2395 M is the molar concentration of sodium nitrate the chemist would have actually prepared.
What is molar concentration?
Molar concentration (also known as molarity, amount concentration, or substance concentration) is a measure of the concentration of a chemical species, specifically a solute in a solution, in terms of amount of substance per unit volume of solution. In chemistry, the most commonly used unit for molarity is the number of moles per liter, denoted by the unit symbol mol/L or mol/dm3 in SI units. A solution with a concentration of 1 mol/L is said to be 1 molar, also known as 1 M.
Homogeneous mixtures or solutions are those with a uniform composition.
Heterogeneous mixtures are those with a non-uniform composition.
The most abundant chemical in the mixture is known as the solvent, and the remaining components are known as solutes.
Molarity, also known as molar concentration, is the number of moles of solute per litre of solution and can be calculated using the following equation:
Molarity = no of moles of solute /L of solution
Given data,
mass of NaNO3 = 4.585 grams
atomic mass of NaNO3 = 84.99 grams/mole
volume of the solution = 200 ml or 0.2 litres
No. of moles = given mass
molar mass
No. of moles = 4.585 = 0.0539 moles
84.99
molarity = No.of moles of solute
volume of solution in litres
molarity = 0.0539 moles
0.2 litres
molarity = 0.2695 M
If the chemist mistakenly makes 225 ml of the solution instead of the 200 ml,
volume = 0.225 litres
molar concentration/ molarity = 0.0539 moles
0.225 litres
molar concentration/ molarity =0.2395 M
Hence, 0.2695 M is molar concentration of sodium nitrate should appear on the label and if the chemist mistakenly makes 225 ml of the solution instead of the 200 ml, 0.2395 M is the molar concentration of sodium nitrate the chemist would have actually prepared.
To know more about molarity from given link
https://brainly.com/question/26873446
#SPJ4
Calculate the molar mass of CC14
Answers
The molar mass of CCl₄ is 154 g/mol
Molar mass is the mass in grams of one mole of a substance and is given by the unit g/mol.
It is calculated by taking the sum of atomic masses of all the elements present in the given formula.
A mole is defined as the amount of substance containing the same number of atoms, molecules, ions, etc. as the number of atoms in a sample of pure 12C weighing exactly 12 g.
Atomic mass of C = 12
Atomic mass of Cl = 35.5
Molar mass = 12 + (35.5 × 4)
= 154 g/mol
Learn more about Molar mass, here:
https://brainly.com/question/12127540
#SPJ1
What is the best definition of solar radiation?Storing Storing of of energy energy in in Earth'Earth's s oceansoceansEnergy Energy from from the the Sun Sun that that reaches reaches EarthEarthMoisture Moisture and and heat heat energy energy in in the the atmosphereatmosphereIncreased Increased evaporation evaporation due due to to warm warm waterwater
Answers
Answer:The solar energy that we receive through solar radiation is directly or indirectly responsible for aspects as important to life as: Photosynthesis in plants; maintaining a planet temperature compatible with life. of the wind. The solar energy that reaches the earth's surface is 10,000 times greater than the energy currently consumed by all
Explanation:
Oxygen gas is collected..)

Answers
The temperature that must be maintained on 0.500 mole of the gas in order to maintain the pressure is 21.76 °C
How do i determine the temperature?From the question given above, the following data were obtained obtained:
Pressure (P) = 1.12 atmVolume of gas (V) = 10.0 LNumber of mole of gas (n) = 0.500 moleGas constant (R) = 0.0821 atm.L/mol KTemperature (T) =?Using the ideal gas equation, we can obtain the temperature as follow:
PV = nRT
1.21 × 10 = 0.500 × 0.0821 × T
12.1 = 0.04105 × T
Divide both sides by 0.04105
T = 12.1 / 0.04105
T = 294.76 K
Subtract 273 to obtain answer in °C
T = 294.76 - 273 K
T = 21.76 °C
Thus, the temperature needed is 21.76 °C
Learn more about temperature:
https://brainly.com/question/23058797
#SPJ1
what is the ph of a 0.0028 m sr(oh)2 solution at 25ºc
Answers
The pH of a 0.0028 M \(Sr(OH)_{2}\) solution at 25°C is approximately 11.75.
1. Determine the concentration of OH- ions:
Since \(Sr(OH)_{2}\) is a strong base and dissociates completely in water, it releases 2 \(OH^{-}\) ions per molecule. Therefore, the concentration of \(OH^{-}\) ions is 2 x 0.0028 M = 0.0056 M.
2. Calculate the pOH:
pOH = -log10(\(OH^{-}\) concentration) = -log10(0.0056) ≈ 2.25
3. Calculate the pH:
Since pH + pOH = 14 at 25°C, the pH can be found by subtracting the pOH from 14:
pH = 14 - 2.25 ≈ 11.75
To know more about pH:
https://brainly.com/question/30257339
#SPJ11
Which is an example of VELOCITY?
Group of answer choices
A dog running at 3 m/s
A migrating bird headed south for the winter at 3 m/s
A deer sprinting at 4 m/s
A person not moving at all
Answers
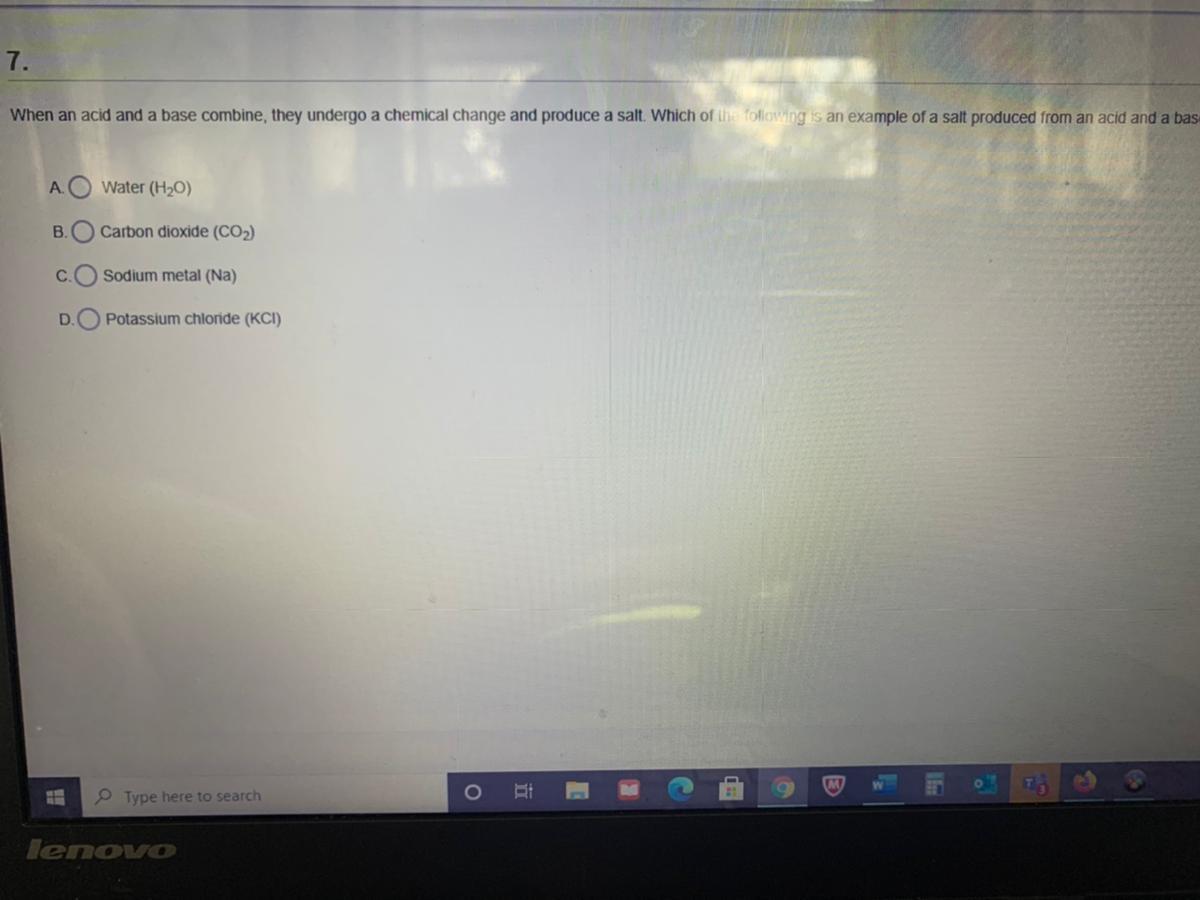
Which property is shared by both acids and bases?
1. changes red litmus paper to blue
2. feels slippery
3. conducts electricity in aqueous solution
4. tastes sour
O 1
2
4
3
Answers
\(\huge{\textbf{\textsf{{\color{navy}{An}}{\purple{sw}}{\pink{er}} {\color{pink}{:}}}}}\)
3. Conducts electricity in aqueous solution.
ThanksHope it helps.How does this equation show that transmutation has taken place? 238 U – 334 Th + He 92 O A. The numbers of neither nucleons nor atoms are conserved. B. One element changes into another. O C. Atoms of different elements are present. O D. The number of nucleons is not conserved.
Answers
Answer:
B. One element changes into another.
Explanation:
We can see from the equation that uranium is changed to thorium and helium. Transmutation is the process by which one element is changed to another through radioactive decay, nuclear bombardment, or other similar processes.
Answer:b
Explanation:

Draw the major organic product of each reaction. Assume a one-to-one ratio of reagents and benzene.For the functional groups added, be sure to draw out all bonds, lone pairs, and formal charges. (Note: the short-cut for NO2 will not be accepted for this question) Did you draw just one compound in each box? Does the organic product contain all bonds, lone pairs and nonzero formal charges? Which electrophile is formed in each route?
Answers
Secondary amine is what it is. Option (B3-dimethylbutan-2-amine )'s is the primary organic product created during the following reaction. Thus, if more hydrogen is added to carbon, we will obtain the main product, according to the Markovnikov Rule.
As a result, the main product produced by adding hydrogen to carbon-1, which has a higher concentration of hydrogen, and adding bromine to carbon-2 is 2-bromopropane. When a reaction follows Markovnikov's rule, a chemical or product is said to be a major organic product. The tertiary alkyl iodide is the largest byproduct of the second process, which transforms alcohol into alkyl iodide, while the secondary alkyl iodide is the minor byproduct.
To learn more about hydrogen here,
https://brainly.com/question/24433860
#SPJ4
identify the various types of solutions. describe the characteristics of each type of solution
Answers
There are several types of solutions, including dilute solutions, concentrated solutions, saturated solutions, unsaturated solutions, and supersaturated solutions. Each type of solution has specific characteristics that distinguish them from one another.
Dilute solutions: These solutions have a small amount of solute dissolved in a larger amount of solvent. They have a low concentration of solute particles. Concentrated solutions: Concentrated solutions have a large amount of solute dissolved in a smaller amount of solvent. They have a high concentration of solute particles. Saturated solutions: Saturated solutions contain the maximum amount of solute that can dissolve in a given amount of solvent at a particular temperature. Any additional solute added will not dissolve. Unsaturated solutions: Unsaturated solutions have not reached their maximum solute capacity and can dissolve more solute. Supersaturated solutions: These solutions contain more solute than what is normally possible at a given temperature. They are achieved by dissolving the solute at a higher temperature and then cooling the solution slowly.
To know more about Dilute : brainly.com/question/28548168
#SPJ11
Which process is not chemical weathering?
A. Acid Rain hitting rocks
B. Cave water (with minerals) breaking down sandstone
C. Salt water flowing over rocks
D. Ice breaking a rock
Answers
Answer:
D because there's no minerals its just the ice
Explanation:
Answer:
Ice breaking a rock
Explanation:

Which of the following is the best explanation for why it is important to follow lab safety guidelines
a. Following laboratory safety guidelines prevents all lab accidents.
b. Following laboratory safety guidelines minimizes the chance of lab ac
C. Following laboratory safety guidelines prevents fires.
d. Following laboratory safety guidelines allows quick response during la
Please select the best answer from the choices provided
Answers
Answer:
B
Explanation:
The main aim of lab rules is to minimize the no of accidents
HELP PLEASE DUE TODAY

Answers
Answer:
Explanation: The invention of the microscope led to the discovery of the cell by Hooke. While looking at cork, Hooke observed box-shaped structures, which he called “cells” as they reminded him of the cells, or rooms, in monasteries. This discovery led to the development of the classical cell theory.
Answer:
Microscopes help see cells up close and if you have for example a Disease they and see what it looks like up close on you cells and discover what it is
Suppose the temperature starts at 60 ∘ F and rises 2 ∘ F every 45 min. Use a table , an equation, and a graph to describe the relationship between the amount of time that has passed in hours and the temperature.
Answers
The linear equation relationship between the time in hours and the temperature is y = (2.67x + 60) ⁰F .
The relationship between the amount of time that has passed in hours and the temperature can be defined using the linear equation. The linear equation should follow this rule
y = mx + c
where y is the effect parameter, m is the gradien, x is cause parameter and c is the initial state of effect parameter.
The gradien can be found by using this formula
m = Δy / Δx
From the question above, the given parameters are
y = temperature
x = time
Δy = 2 ⁰F
Δx = 45 min = 0.75 hour
c = 60 ⁰F
Find the gradien
m = Δy / Δx
m = 2 / 0.75
m = 2.67
The relationship between temperature and time should be
y = mx + c
y = (2.67x + 60) ⁰F
Find more on linear equation at: https://brainly.com/question/2030026
#SPJ4
What are the two types of carbohydrates and food sources?.
Answers
Answer: simple and complex.
Explanation:These are also called simple sugars. They're found in refined sugars, like the white sugar you see in a sugar bowl. If you have a lollipop, you're eating simple carbs.
What started universe (Science)
Answers
Answer:
Big bang theory
Explanation:
Our universe began with an explosion of space itself - the Big Bang. Starting from extremely high density and temperature, space expanded, the universe cooled, and the simplest elements formed. Gravity gradually drew matter together to form the first stars and the first galaxies.
Answer:
The Big Bang
Explanation:
The Big Bang was the moment 13.8 billion years ago when the universe began as a tiny, dense, fireball that exploded. Most astronomers use the Big Bang theory to explain how the universe began.
A sample of gas has a volume of 526 mL at 346 mmHg and 35.0°C. Determine the moles of gas.
Answers
Answer:
The moles of gas is 0.009474 moles.
Explanation:
Given that,
Volume = 526 mL
Pressure = 346 mmHg
Temperature = 35.0°C
We need to calculate the moles of gas
Using formula of ideal gas
\(n=\dfrac{PV}{RT}\)
Where, P = pressure
V = volume
R = gas constant
T = temperature
Put the value into the formula
\(n=\dfrac{\dfrac{346}{760}\times0.526}{0.08206\times(35+273)}\)
\(n=0.009474\ moles\)
Hence, The moles of gas is 0.009474 moles.
For every 6 mols of H2 how many mols of h20 will be produced?
For every 2 mols of H2 how many mols of h20 will be produced?
For every 5.67 mols of H2 how many mols of h20 will be produced?
Answers
The moles would be 12 moles, 4 moles and 11.34 moles
How to solve for the molesWhen hydrogen gas (H2) reacts with oxygen gas (O2) to produce water (H2O), the balanced chemical equation is:
2H2 + O2 -> 2H2O
So,
For every 6 moles of H2, 12 moles of H2O will be produced
For every 2 moles of H2, 4 moles of H2O will be produced
For every 5.67 moles of H2, 11.34 moles of H2O will be produced.
Read more on the moles here:https://brainly.com/question/15356425
#SPJ1
Which is the best example and explanation that a physical change has occurred?
Answers
A 4.80g piece of magnesium displaces 2.76 mL of water when it is placed in a graduated cylinder. What is the density of magnesium?
Answers
Answer:
6
What do AIA, SAG, AFP and AMA all stand for? Select four options.
American Institute of Architects
American Society of Mechanical Engineers
American Medical Association
Screen Actors Guild
Association for Finance Professionals
Professional Colleges
How much heat will be absorbed in
boiling 5 grams of ice at 100°c and
at 1 atm?
Answers
Source
Trust me bro
What is the bond angle in carbon dioxide, CO2?
Answers
There is a Canvas AUDIO tutorial available for this problem. Neglecting activities, calculate the pH of a solution containing 0.018 M NaOH plus 0.0120 M LINO3. 4.0 12.26 Using activities, re-calculate the actually pH of the solution. 4.9 12.2 X Check the number of significant figures. More Information
Answers
The pH of the solution, neglecting activities, is approximately 12.26.
To calculate the pH of the solution, we need to consider the dissociation of water, as well as the dissociation of the solutes NaOH and LiNO3. Let's go through the steps:
Step 1: Identify the ions present in the solution:
The solution contains NaOH and LiNO3. NaOH dissociates into Na+ and OH- ions, while LiNO3 dissociates into Li+ and NO3- ions.
Step 2: Write the balanced chemical equations for the dissociation of NaOH and LiNO3:
NaOH dissociates as follows:
NaOH -> Na+ + OH-
LiNO3 dissociates as follows:
LiNO3 -> Li+ + NO3-
Step 3: Calculate the concentration of each ion:
Given that the concentration of NaOH is 0.018 M and LiNO3 is 0.0120 M, the concentration of Na+ and Li+ ions is equal to their respective initial concentrations.
For NaOH:
[Na+] = 0.018 M
[OH-] = 0.018 M (since NaOH dissociates in a 1:1 ratio)
For LiNO3:
[Li+] = 0.0120 M
[NO3-] = 0.0120 M (since LiNO3 dissociates in a 1:1 ratio)
Step 4: Calculate the concentration of H+ ions:
Since we have the concentrations of OH- ions, we can use the fact that water undergoes auto-ionization to determine the concentration of H+ ions.
[H+] = Kw / [OH-]
At 25°C, the value of Kw (the ion product of water) is approximately 1.0 x 10^-14.
[H+] = (1.0 x 10^-14) / (0.018)
Using a calculator, we find that the concentration of H+ ions is approximately 5.56 x 10^-13 M.
Step 5: Calculate the pH:
pH = -log[H+]
pH = -log(5.56 x 10^-13)
Using a calculator, we find that the pH of the solution neglecting activities is approximately 12.26.
Now, let's consider the re-calculation of pH using activities:
To re-calculate the pH using activities, we need additional information, such as activity coefficients for the ions present. However, the given question does not provide the necessary activity coefficients or any other information required for activity calculations. Therefore, it is not possible to re-calculate the actual pH of the solution using activities without that information.
In summary:
The actual pH of the solution considering activities cannot be determined without additional information, such as activity coefficients.
Learn more about ph here:
https://brainly.com/question/30761746
#SJP11
When comparing two similar chemical reactions, the reaction with
the smaller activation energy (Ea) will have…
Answers
The activation energy denoted by Ea is referred to as the minimum energy or the threshold energy required for a reaction to occur. When reactant molecules overcome the repulsion energy resulting in the collision of outer electrons by optimum kinetic energy. Higher the activation energy more easy for the reaction to take place.
When comparing two similar chemical reactions, the reaction with the smaller activation energy (Ea) because the activation energy is lower, the transition of the reactant molecules into the product species requires less energy. Accordingly, the pace of the response will be higher.
Learn more about activation energy, here:
https://brainly.com/question/28384644
#SPJ4
What color is the acetic acid plus methyl orange after addition of sodium acetate solution and what does this tell you about where the equilibrium?
Answers
The color of acetic acid plus methyl orange after the addition of sodium acetate solution is yellow-orange, which tells us that the equilibrium has shifted to the right.
What is the color of acetic acid plus methyl orange after the addition of sodium acetate solution, and what does this tell you about where the equilibrium lies?
The indicator methyl orange changes color from red to yellow when the pH goes from below 3.1 to above 4.4, making it a good indicator for the titration of acetic acid with a strong base like NaOH.
Sodium acetate and acetic acid react to form a buffer solution that resists changes in pH when a strong acid or base is added.
Sodium acetate and acetic acid are in equilibrium, with the acetic acid donating protons to the acetate ion to create the acetate anion and the hydronium ion. The equilibrium between acetic acid and acetate ion is the equilibrium of the system, which can be represented as CH3COOH + H2O ⇌ H3O+ + CH3COO-.
After the addition of sodium acetate solution, the color of acetic acid plus methyl orange changes to yellow-orange. This shift towards the right shows that the equilibrium has shifted to the right, indicating that more acetate ions and hydronium ions have formed from acetic acid molecules as a result of the reaction.
As a result, the pH of the solution has decreased, making it more acidic. The acidity of the solution has been improved by the formation of more hydronium ions, which can react with the added strong base to produce water.
To know more about the Sodium acetate https://brainly.com/question/12924347
#SPJ11
Match the scientist to his contribution to the atomic theory. (3 points)
Answers
Answer: Thomson - Electrons
Rutherford - Nucleus
Bohr - Electron energy levels
Explanation: I just took the quiz
Answer:
Answer: Thomson - Electrons
Rutherford - Nucleus
Bohr - Electron energy levels
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
Draw a Lewis structure for NO4-3 then label the formal charges on each atom and finally propose a structure to minimize the formal charge.
Answers
The Lewis structure for NO4-3 was labelled the formal charges on each atom
\(\left[\begin{array}{ccc} *&*&*\\*&O&*\\*&*&*\end{array}\right]\)
| {\(\left[\begin{array}{ccc} *&*&*\\*&O&*\\&&\end{array}\right]\) =N- \(\left[\begin{array}{ccc} *&*&*\\*&O&*\\*&*&*\end{array}\right]\)}^3-
|
\(\left[\begin{array}{ccc} *&*&*\\*&O&*\\*&*&*\end{array}\right]\)
What is a Lewis structure?A Lewis structure, also known as a Lewis dot diagram, is a way to represent the bonding and non-bonding electrons in a molecule. It is named after Gilbert N. Lewis, who introduced it in 1916. A Lewis structure shows the chemical symbol of each atom in the molecule and the electrons that are involved in the chemical bonds. It is used to predict the molecular geometry, reactivity, and other chemical properties of a molecule.
What is formal charge on nitrogen and oxygen atoms?The formal charge on the Nitrogen atom is 0 and each Oxygen atom has a formal charge of -1. A structure that minimizes the formal charge is one in which the formal charges on all the atoms are as close to zero as possible. This can be achieved by using the electrons from the central Nitrogen atom (N) to form single bonds with each Oxygen atom (O)
To learn more about lewis structure visit:
brainly.com/question/20300458
#SPJ4
use the formal charge for each atom in each of the lewis structures given, to predict which one more likely shows the correct connectivity for nof.
Answers
Use the formal charge for each atom in each of the lewis structures given, to the one more likely shows the correct connectivity for NOF is
O = N - F.
formal charge for N = O - F
formal charge = valence electrons - non bonding electrons - (1/2) bonding
electrons
formal charge for N = 5 - 4 - (1/2) 4
= -1
formal charge for O = 6 - 2 - (1/2)6
= +1
formal charge for F = 7- 6 - (1/2)2
= 0
formal charge for O = N - F
formal charge for N = 5 - 2 - (1/2)6
= 0
formal charge for O = 6 - 4 - (1/2) 4
= 0
formal charge for F = 7 - 6 - (1/2)2
= 0
Thus, Use the formal charge for each atom in each of the lewis structures given, to the one more likely shows the correct connectivity for NOF is O = N - F because in this structure all atoms have zero formal charge.
To learn more about formal charge here
https://brainly.com/question/28811018
#SPJ4