SN1 reactions usually proceed with: Group of answer choices complete inversion at the center undergoing substitution. Slightly more inversion than retention at the center undergoing substitution. Equal amounts of inversion and retention at the center undergoing substitution. Slightly more retention than inversion at the center undergoing substitution. Complete retention at the center undergoing substitution
Answers
SN1 reactions usually proceed with equal amounts of inversion and retention at the center undergoing substitution.
In SN1 (Substitution Nucleophilic Unimolecular) reactions, the stereochemistry of the reaction is not generally characterized by equal amounts of inversion and retention at the center undergoing substitution. Instead, SN1 reactions typically lead to racemization or a mixture of stereoisomers.
In an SN1 reaction, the reaction proceeds in two steps. First, the leaving group departs from the substrate, generating a carbocation intermediate. Then, the nucleophile attacks the carbocation, resulting in the formation of the substitution product.
The key factor determining the stereochemistry of SN1 reactions is the nature of the carbocation intermediate. Carbocations are planar and lack stereochemistry.
As a result, the nucleophile can approach the carbocation from either side, leading to the formation of a mixture of stereoisomers or racemization.
Therefore, SN1 reactions typically result in the formation of both inverted and retained products, along with the possibility of racemization. The specific distribution of stereoisomers will depend on factors such as the nature of the nucleophile, the leaving group, and the reaction conditions.
To learn more about retention, refer below:
https://brainly.com/question/29709076
#SPJ11
Related Questions
A mixture of krypton and carbon dioxide gases, at a total pressure of 798 mm Hg, contains 14.2 grams of krypton and 6.92 grams of carbon dioxide. What is the partial pressure of each gas in the mixture? P Kr = ______ mmHg P CO2 = _______ mmHg
Answers
The partial pressure of krypton is 415.6 mmHg and the partial pressure of carbon dioxide is 382.4 mmHg.
To find the partial pressure of each gas in the mixture, we need to use the mole fraction of each gas.
First, we need to find the moles of each gas in the mixture:
moles of Kr = 14.2 g / 83.8 g/mol = 0.1699 mol
moles of CO2 = 6.92 g / 44.01 g/mol = 0.157 mol
Next, we need to find the total moles in the mixture:
total moles = moles of Kr + moles of CO2 = 0.1699 mol + 0.157 mol = 0.3269 mol
Now we can find the mole fraction of each gas:
mole fraction of Kr = moles of Kr / total moles = 0.1699 mol / 0.3269 mol = 0.5198
mole fraction of CO2 = moles of CO2 / total moles = 0.157 mol / 0.3269 mol = 0.4802
Finally, we can use the mole fractions to calculate the partial pressure of each gas:
partial pressure of Kr = mole fraction of Kr x total pressure = 0.5198 x 798 mmHg = 415.6 mmHg
partial pressure of CO2 = mole fraction of CO2 x total pressure = 0.4802 x 798 mmHg = 382.4 mmHg
Therefore, the partial pressure of krypton is 415.6 mmHg and the partial pressure of carbon dioxide is 382.4 mmHg.
To know more about partial pressure refer here:
https://brainly.com/question/31214700
#SPJ11
Does knowing the ratio of masses of the elements in a compound lead to the unique chemical identity of the compound?
Answers
Isomers are compounds that have the same molecular formula but different arranged differently of the atoms.
For example
So even if they have the same proportion of their atoms, the chemical identity is different
The answer is option B: no because more than one compound can have the same ratio of masses of elements if the atoms are arranged differently.

For the below equation, balance and determine the ratios listed below:

Answers
Answer:
The balanced chemical equation is:
\(2Al_2O_3\operatorname{\rightarrow}4Al+3O_2\)- Ratio of Al2O3 to O2: 2:3R
- Ratio of Al2O3 to Al: 2:4R
- Ratio of Al to O2: 4:3R
Explanation:
To balance the chemical equation, it is necessary to have the same amount of elements on the reactant side as on the product side:
\(2Al_2O_3\rightarrow4Al+3O_2\)Now we know that the reaction is balanced, because on the reactant side and on the products side there are:
- 4 Al
- 6 O
Now that the equation is balanced, we can write the ratios with the stoichiometry of the reaction.
What is vocational training
Answers
Answer:
Vocational training refers to instructional programs or courses that focus on the skills required for a particular job function or trade. Vocational training education prepares students for specific careers, disregarding traditional, unrelated academic subjects.
what happens when zinc is reacted with sulphuric acid?
Answers
Answer:
formation of zinc sulphate which is soluble
Explain biomass combustion and energy recovery using grate
furnace or fluidized bed systems
Answers
Biomass combustion is referred to as a process in which organic materials are burnt and their remains are used to produce energy.
The process of combustion is very simple it refers to the burning of biomass which include wood, farm waste, and crops which are further used to produce or generate energy in the form of electricity and also heat, it can be termed as renewable energy that utilized the energy of biomass to produce another form of energy.
The Grate furnace method is one of the common methods used for biomass combustion and comprises several steps for the recovery of energy.
The first step consists of drying up the biomass by removing all the moisture using heat. The next step includes the production of flames and heat by combusting hydrogen present in it. After that, the remaining solid waste will undergo combustion in the presence of oxygen.
The last step includes the disposal of ash which gets accumulated due to incombustible materials like sand.
Learn more about combustion
https://brainly.com/question/23992512
What is the total number of atoms in a 30. gram sample of neon?
Answers
1.5 mole or 1.5 total number of atoms are there in a 30. gram sample of neon .
What do you mean by the mole concept ?
The mole concept is a convenient method of expressing the amount of a substance.
Mole is the amount of substance that contains as many elementary entities as there are atoms in exactly 12 grams of the carbon−12 isotope.
1 mole=6.022×1023 particles of matter of any chemical substance.
To calculate the total number of atoms in 30 gm sample of neon-
Given,
Mass of the substance =30 gm
Molar mass of neon = 20.17 a.m.u
n(no. of atoms )=?
We know number of atoms is given -
n=mass/molar mass
n=30/20.17
n=1.48≈1.5 mole
The total number of atoms in a 30 gm sample of neon will be 1.5 mole .
Learn more about mole concept , here:
https://brainly.com/question/22540912
#SPJ6
What is the percentage of Aluminum in Aluminum Sulfate?
Answers
Answer:
342. 15 g/mol is the mass
Al Aluminium 26.98g 2 atoms per mole = 15.7716%
S Sulfur 32.06g 3 atoms per mole = 28.1151%
O Oxygen 15.99g 12 atoms per mole = 56.1133%
Explanation:
Hope this helps
Answer:
15,7%
Explanation:
What is the percentage of Aluminum in Aluminum Sulfate?
Aluminum Sulfate
is Al2 (SO4)3
its molar mass is
(2 X 27) + (3 X 96) = 54 + 288 = 344
THE % Al IS (54 /344) X 100 = 15,7%
the mass of the sun
what is the mass of the sun
Answers
Answer:
1.989 × 10^30 kg
Explanation:
Hope this helps! :3
☁️ Answer ☁️
A solar mass is the mass of the sun. Or, more precisely, it's 1.989 x 10^30 kilograms — about 333,000 Earths. Astronomers use a solar mass as a basic unit of mass.
Dear fool, you will always be a fool.
Happy April Fools Day.
Hope it helps.
Have a nice day noona/hyung
A camel eats 18.3 kg of Bermudagrass hay that is 14.7 %
CP on a dry matter basis. If the DM percentage of the hay is 83.4
%, how much protein did the camel consume?
Answers
The camel consumed approximately 2.24 kg of protein from the Bermudagrass hay.
To calculate the amount of protein the camel consumed, we need to consider the dry matter basis of the hay. Here's how you can calculate it:
Calculate the dry matter weight of the hay:
Dry Matter Weight = Total Weight of Hay × Dry Matter Percentage
Dry Matter Weight = 18.3 kg × (83.4/100)
Dry Matter Weight = 18.3 kg × 0.834
Dry Matter Weight = 15.2442 kg
Calculate the protein content in the dry matter;
Protein Content = Dry Matter Weight × Protein Percentage
Protein Content = 15.2442 kg × (14.7/100)
Protein Content = 15.2442 kg × 0.147
Protein Content = 2.2414194 kg
Therefore, the camel consumed approximately 2.24 kg of protein from the Bermudagrass hay.
To know more about Bermudagrass here
https://brainly.com/question/30516027
#SPJ4
Is oxygen and potassium an ionic bond?
Answers
Ionic bond: Metal & Nonmetal
Brainliest is appreciated
can anyone please help with this?

Answers
Answer:
It's the first or the second answer.
Explanation:
21(3d − 4) + 100 = 58 State the solution. (If all real numbers
are solutions, enter REALS. If there is no solution, enter NO
SOLUTION.)
Answers
The solution to the equation and value of variable 21(3d - 4) + 100 = 58 is d = 2/3.
Solve the equation 21(3d - 4) + 100 = 58, we can begin by simplifying and isolating the variable:
21(3d - 4) + 100 = 58
Distribute 21 to the terms inside the parentheses:
63d - 84 + 100 = 58
Combine like terms:
63d + 16 = 58
Subtract 16 from both sides:
63d = 42
Divide both sides by 63:
d = 42/63
Simplifying the fraction gives:
d = 2/3
The solution to the equation is d = 2/3.
The solution to the equation 21(3d - 4) + 100 = 58 is d = 2/3. By simplifying the equation, we find that dividing both sides by 63 results in the solution of d = 2/3, which satisfies the original equation.
To know more about variable refer here
https://brainly.com/question/15078630#
#SPJ11
What is the correct representation for the sun shell with n=2 and I = 1?
Answers
Answer:
2p is the correct representation
Explanation:
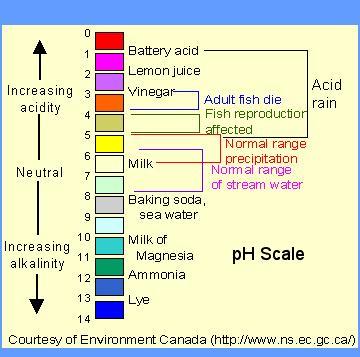
you have two solutions. one of them has a ph of 5. the other has a ph of 2. what is the magnitude change of h ion concentration in the second solution compared to the first?
Answers
The magnitude change of H ion concentration in the second solution compared to the first is 1000-fold (10³).
The pH scale measures the acidity or basicity of a solution based on the concentration of H+ ions present. It is a logarithmic scale, which means that each change of one pH unit corresponds to a tenfold change in H⁺ concentration.
Therefore, the difference of three pH units between the two solutions corresponds to a 10³ or 1000-fold difference in H⁺ ion concentration. This means that the second solution with a pH of 2 is much more acidic than the first solution with a pH of 5, and its H⁺ ion concentration is 1000 times higher.
In general, the pH scale provides a convenient way to compare the relative acidity or basicity of different solutions, and it has many important applications in chemistry, biology, and other fields.
To learn more about pH scale visit: https://brainly.com/question/15075648
#SPJ4
How would you synthesize the following compounds from benzene using reagents from the table? Reagents NaOH / H2O 2. H3O NBS / (PhCO2)2 3. CH2Cl 4. AICl2 5. Ci2 6. CH2Cl 7. Br2 8. FeBra 9. Cl2 10. FeCl; 11. CO2 12. NaCN 13. KMnO4/H2O+ 14. Mg / dry ether a) p-Nitrobenzoic acid _____
b) m-Nitrobenzoic acid' ______
Answers
The correct answer is a) p-Nitrobenzoic -acid can be synthesized from benzene using the following steps:
1. Nitration of benzene to form nitrobenzene, using a mixture of concentrated nitric acid and concentrated sulfuric acid (HNO3/H2SO4).
2. Oxidation of nitrobenzene to form p-nitrobenzoic acid, using a mixture of NaOH and H2O2.
b) m-Nitrobenzoic acid can be synthesized from benzene using the following steps:
1. Nitration of benzene to form nitrobenzene, using a mixture of concentrated nitric acid and concentrated sulfuric acid (HNO3/H2SO4).
2. Bromination of nitrobenzene to form m-Bromo nitrobenzene, using NBS and (PhCO2)2.
Reduction of m-Bromo nitrobenzene to form m-nitrobenzene, using Mg/dry ether.
Oxidation of m-nitrobenzene to form m-nitrobenzoic acid, using KMnO4/H2O+.
Note: The synthesis of both compounds involves nitration of benzene, which is a highly exothermic reaction and should be carried out with caution. It is important to follow the proper safety protocols and handle the reagents carefully.
To learn more about Nitrobenzene click the link below
brainly.com/question/13190784
#SPJ4
NEED HELP ASAP WILL GIVE BRAINIEST

Answers
Which one of the following solvents is commonly used in the extraction of and indicator from flowers?
Answers
Ph4 is greater than ph6?.
Answers
Answer:
Explanation: For example, pH 4 is ten times more acidic than pH 5 and 100 times (10 times 10) more acidic than pH 6. The same holds true for pH values above 7, each of which is ten times more alkaline (another way to say basic) than the next lower whole value.
HOPE THAT HELPS
hi can someone please help me answer this the question is in pictures

Answers
2. East
3. Warm stormy weather
2 Three elements are represented by the letters X,
Y and Z. One atom of Z is two times heavier
than one atom of Y. One atom of Y is three
times heavier than one atom of X. What is the
relative atomic mass of Z if the relative atomic
mass of X is 31?
Answers
Answer:
Here is my answer. check it out

An old refrigerator is rated at 500 W how many kilowatt hours of electric energy what does refrigerator use in 30 days assume the refrigerator is running 12 hours per day
Answers
The refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
To calculate the kilowatt-hours (kWh) of electric energy used by the refrigerator in 30 days, we need to multiply the power rating by the total running time.
Given:
Power rating of the refrigerator = 500 W
Running time per day = 12 hours
Number of days = 30
First, we need to convert the power rating from watts to kilowatts:
Power rating = 500 W / 1000 = 0.5 kW
Next, we calculate the total energy used in kilowatt-hours (kWh) over the 30-day period:
Energy used = Power rating × Running time × Number of days
Energy used = 0.5 kW × 12 hours/day × 30 days
Energy used = 180 kWh
Therefore, the refrigerator would use 180 kilowatt-hours (kWh) of electric energy over the course of 30 days, assuming it runs for 12 hours each day.
For more question on energy
https://brainly.com/question/29339318
#SPJ8
How many meters are in 37 cm?
0.37 m
0.037 m
3700 m
370 m
Answers
Answer:
the correct answer is 0.37m
Explanation:
37/100 = 0.37m
Answer:
How many meters are in 37 cm?
In 37 cm - 0.37 m
>3
identify the best reagents to convert 1-hexyne into (e)-1,2-dibromo-1-hexene.select answer from the options belowxs br2, ccl41 equiv hbr, roorxs hbr1 equiv. br2, ccl41 equiv hbr
Answers
The best reagents to convert 1-hexyne into (e)-1,2-dibromo-1-hexene are 1 equiv. Br2 in CCl4, followed by NaOH to convert the mixture of (Z)- and (E)-isomers to the desired (E)-isomer.
This reaction is called the Vicinal Dibromination reaction. Option A: xs Br2 in CCl4 is a good choice of reagents, but it will give a mixture of (Z)- and (E)-isomers. Option B: 1 equiv. HBr will result in the formation of (Z)-1-bromo-1-hexene. Option C: ROOR is a radical initiator and will not result in the desired product.
To know more about different reagents and isomers : https://brainly.com/question/29713522
#SPJ11
Which graph best represents a car traveling down the freeway at a constant
speed? *

Answers
Answer:
C
Explanation:
on a graph, the line would be straight if it is constant speed
Answer:
c
Explanation:
its staying at the same speed in a straight line
A student needed to nitrate benzoic acid. Benzoic acid is a solid at standard conditions so the student dissolved the benzoic acid in benzene and then added the nitric acid/sulfuric acid reagent system. When the student subjected the isolated product to spectroscopic techniques to verify that she had successfully nitrated the benzoic acid she found that the product was not consistent with nitrobenzoic acid (neither ortho, meta nor para). The spectroscopic data was consistent with a nitrated product.
(a) Draw the skeletal structure of the nitrated product.
(b) Since the student was not successful using benzene as the solvent, she decided to try to nitrate benzoic acid using a different solvent. Select all of the solvents that would probably lead to major success. (Select all that apply.)
bromobenzene
toluene
o-xylene
nitrobenzene
benzaldehyde
Answers
The solvents that would likely lead to major success in nitration of benzoic acid are toluene and nitrobenzene.
What is the solvent?Benzoic acid can be nitrated under favorable conditions using toluene, which is frequently employed as a solvent in nitration operations. Being a nitro-substituted benzene compound, nitrobenzene can also work well as a solvent for nitrating benzoic acid.
In nitration reactions, bromobenzene and o-xylene are not frequently employed as solvents, and benzaldehyde is not a good choice either.
Learn more about nitration:https://brainly.com/question/5346392
#SPJ4

Drag and drop the words that accurately complete the chart below. Example a lion and a cheetah mistletoe on a tree a coyote eating a rabbit a remora and a shark clownfish and anemone parasitism friendship competition Type of Symbiosis mutualism 1:10 predation relationship commensalism collaboration alliance

Answers
Answer:
Lion and cheetah - Competition
Mistletoe on a tree - Parasitism
Coyote eating rabbit- Predatation
Remora and Shark - Mutualism
Clownfish and Anemone - Relationship
Explanation:
how is matter and energy conserved when liquid is frozen
Answers
Answer:
The big energy change when water freezes is in the potential energy of interactions between the water molecules.
Explanation:
I am not to sure if this is correct, but I hope it helps in some way.
label the ions so that the unit cell represents the structure of an inverse spinel, cofe2o4.
Answers
Eight tetrahedral sites are labeled with Fe³⁺ ions and four octahedral sites are labeled with Co²⁺ ions.
To represent the inverse spinel structure of CoFe₂O₄ in a unit cell, we need to label the ions based on their positions in the crystal structure.
In the inverse spinel structure, one-half of the octahedral sites are occupied by divalent metal ions, while one-half of the tetrahedral sites and the remaining octahedral sites are occupied by trivalent metal ions. The chemical formula of CoFe₂O₄ suggests that the cobalt (Co) ions occupy one-half of the octahedral sites, while the iron (Fe) ions occupy both the tetrahedral and the remaining half of the octahedral sites.
Therefore, we can label the ions in the unit cell of CoFe₂O₄ as follows:
Eight tetrahedral sites are labeled with Fe³⁺ ions.
Four octahedral sites are labeled with Co²⁺ ions.
Four octahedral sites are labeled with Fe³⁺ ions.
The unit cell of CoFe₂O₄ can be represented by the following diagram, where the small spheres represent Co²⁺ ions, and the large spheres represent Fe³⁺ ions:
Note that this is a simplified representation of the unit cell, as it only shows one layer of ions. In reality, the structure of CoFe₂O₄ is three-dimensional, and the unit cell contains many more ions.
The correct question is :
Label the ions so that the unit cell represents the structure of an inverse spinel, CoFe₂O₄. (Picture below)
To know more about tetrahedral follow the link:
https://brainly.com/question/14007686
#SPJ4


Mention and discuss briefly the adverse effects of chemistry.
Answers
Depending on the chemical, these longer-term health effects might include:
organ damage. weakening of the immune system. development of allergies or asthma. reproductive problems and birth defects. effects on the mental, intellectual or physical development of children. cancer.