silver metal reacts with sulfur to form silver sulfide according to the following balanced equation: if 0.700 moles ag is reacted with 10.0 g s, is sulfur or silver the limiting quuizletreactant? sulfur is the limiting reactant how many grams of ag2s will be produced?
Answers
Sulfur is the limiting reactant, and 77.3 g of \(Ag_{2} S\) will be produced. Ag is in excess.
The reasonable synthetic condition for the response is:
2 Ag + S - > \(Ag_{2} S\)
To figure out which reactant is restricting, we really want to look at the quantity of moles of Ag and S present in the given sums.
Moles of Ag = 0.700 moles
Moles of S = 10.0 g/32.06 g/mol = 0.312 moles
The stoichiometric proportion of Ag to S is 2:1. Along these lines, 2 moles of Ag respond with 1 mole of S.
Since we have just 0.312 moles of S accessible, this implies that Ag is in abundance and S is the restricting reactant. This end can likewise be reached by contrasting the stoichiometric proportions of Ag and S in the response. To compute how much \(Ag_{2} S\) created, we really want to utilize the mole proportion of \(Ag_{2} S\) to S, which is 1:1.
Since we have 0.312 moles of S, we can deliver 0.312 moles of \(Ag_{2} S\).
The molar mass of \(Ag_{2} S\) is 247.8 g/mol. Subsequently, the mass of \(Ag_{2} S\)delivered is:
Mass of \(Ag_{2} S\) = 0.312 moles * 247.8 g/mol = 77.3 g
Hence, when sulfur is the restricting reactant, 77.3 grams of \(Ag_{2} S\) will be delivered. It's vital to take note of that how much silver (Ag) present in the response is more prominent than the sum expected for complete response with the accessible sulfur (S). In this way, a portion of the Ag will remain unreacted toward the finish of the response.
To learn more about limiting reactant, refer:
https://brainly.com/question/22080452
#SPJ4
Related Questions
A 7.12×10^−4mol sample of KOH is dissolved in water to make up 50.0mL of solution. What is the pH of the solution? Round the answer to three significant figures.
Select the correct answer below:
12.2
1.85
15.85
10.9
Answers
The correct answer is 12.9.
First, we need to calculate the concentration of the KOH solution:
Molarity = moles of solute / volume of solution in liters
Converting the volume of solution to liters:
50.0 mL = 50.0 × \(10^-3\) L = 0.0500 L
Converting the number of moles to concentration:
Molarity = 7.12×\(10^-4\) mol / 0.0500 L = 0.0142 M
Next, we can use the fact that KOH is a strong base to find the concentration of hydroxide ions in the solution. In a 0.0142 M solution of KOH, the concentration of hydroxide ions is also 0.0142 M.
pH = 14 - log[OH-]
pH = 14 - log(0.0142) = 12.85
Rounding to three significant figures gives a pH of 12.9.
Therefore, the correct answer is 12.9.
To know more about concentration refer here
brainly.com/question/3045247#
#SPJ11
There are 4.78 g of dry LICIO4 and
2.43 g H₂O in the sample.
Step 2: Determine the moles of water and anhydrous compound.
Li = 6.94 g/mol, Cl = 35.45 g/mol,
H = 1.01 g/mol, O = 16.00 g/mol
How many moles of LICIO4 are present?
[?] mol LICIO4
Answers
Answer:
The number of moles of H₂O is 0.135 mol.
The number of moles of LiClO₄ is 0.0449 mol.
Explanation:
Mole is an important standard unit used for the measurement of large quantities of atoms, molecules, or other particles. One mole is equal to 6.022×10²³ units.
The number of moles of a substance is calculated by:
\(\frac{mass of substance}{molecular weight of substance}\)
To find the number of moles of H₂O:
Mass of H₂O in the sample = 2.43g
The molecular weight of H₂O = 18.02g
Number of moles = \(\frac{2.43}{18.02}\) = 0.135 mol.
To find the number of moles of LiClO₄:
Mass of LiClO₄ given = 4.78g
The molecular weight of LiClO₄ = 106.39g
Number of moles = \(\frac{4.78}{106.39}\) = 0.0449 mol.
Learn more about Moles here:
https://brainly.com/question/855186
#SPJ2
Answer:
0.0449
Explanation:
No probelm
Students mixed two liquids in a beaker and listed their observations. Observations Liquid 1 was colorless. Liquid 2 was colorless. The mixture of liquids 1 and 2 formed a colorless solution. Small, solid particles formed and fell to the bottom of the beaker. Based on these observations, which statement contains the best evidence that a chemical reaction occurred?
Answers
The best proof that a chemical reaction has occurred based on the observations above is: Small, solid particles formed and fell to the bottom of the beaker.
Chemical reactions occur when the chemical composition of a substance changes.
What is the best proof that a chemical reaction has occurred based on the following observations?
Students mixed two liquids in a beaker and listed their observations. Observations Liquid 1 was colorless. Liquid 2 was colorless. The mixture of liquids 1 and 2 formed a colorless solution. Small, solid particles formed and fell to the bottom of the beaker.
When two liquids are mixed and a solid forms, that is the best indication that a chemical reaction has occurred. This indicates a precipitation reaction has occurred in which an insoluble substance forms from a solution. This chemical reaction occurs when two aqueous solutions are mixed and the resulting solution becomes a solid compound called a precipitate. Precipitation reactions are one type of double replacement reaction that occurs in water solutions.
Therefore, the best proof that a chemical reaction has occurred based on the observations above is: Small, solid particles formed and fell to the bottom of the beaker.
To learn more about chemical reaction visit
https://brainly.com/question/22817140
#SPJ11
In Experiment 2 a gas is produced at the negative electrode.
Name the gas produced at the negative electrode.
Answers
In Experiment 2, the gas produced at the negative electrode is typically hydrogen (H2).
\(\huge{\mathfrak{\colorbox{black}{\textcolor{lime}{I\:hope\:this\:helps\:!\:\:}}}}\)
♥️ \(\large{\underline{\textcolor{red}{\mathcal{SUMIT\:\:ROY\:\:(:\:\:}}}}\)
Complete the following table. Round each of your answers to 3 significant digits. energy content when eaten food cal kcal kJ Х a cup of chicken noodle soup 4 7.50 X 10 75.0 314. a slice of cooked bacon 36.0 0 a 12-ounce soft drink - 1 1.50 X 10 150. 628.
Answers
A cup of chicken noodle soup has 4 calories (cal), 7.50 x 10³ kilocalories (kcal), and 314 kilojoules (kJ) of energy content when eaten.
A slice of cooked bacon has 36 calories (cal), 0 kilocalories (kcal), and 151 kilojoules (kJ) of energy content when eaten. A 12-ounce soft drink has 1.5 x 10^2 calories (cal), 628 kilojoules (kJ) of energy content when eaten.
Calories (cal) measure the amount of energy released from the food when it is metabolized by the body. Kilocalories (kcal) are a metric unit of energy equivalent to 1,000 calories. Kilojoules (kJ) is another metric unit of energy, and it is equal to 4.184 kilocalories.
In general, it takes about 4.184 kilojoules of energy to raise the temperature of one kilogram of water by one degree Celsius. This is why kilojoules are sometimes used to measure the energy content of food.
To know more about kilocalories click on below link:
https://brainly.com/question/28460095#
#SPJ11
I am mixing vinegar and baking soda in a bottle. If I want to be sure that Conservation of Mass is followed, what should I make sure to do?
Answers
Law of conservation of mass was crucial to progression of chemistry, as it helped scientists understand that substances did not disappear as result of reaction.
What is conservation of mass?Law of conservation of mass explains that in chemical reaction mass is neither created nor destroyed.
To ensure Conservation of Mass is followed when mixing vinegar and baking soda in a bottle, you should make sure to do the following:
Weigh the bottle and its contents before adding vinegar and baking soda. This will give the initial mass of system.
Carefully measure the amounts of vinegar and baking soda you add to bottle.
Quickly and securely cap the bottle after adding baking soda to avoid any gas from escaping.
Weigh the bottle and its contents again after reaction has completed and the gas has stopped escaping. This will give the final mass of the system.
Compare the initial and final masses of system. They should be equal within experimental error.
To know more about conservation of mass, refer
https://brainly.com/question/15289631
#SPJ1
If your end product is 1.5 moles of KMnO4, how many moles of manganese oxide were used in the reaction?
The equation for the production of potassium permanganate is as follows:
2 MnO2 + 4 KOH + O2 → 2 KMnO4 + 2 KOH + H2
You must show all work
Answers
Answer:
1.5 moles of KMnO4 will be produced from 1.5 moles of MnO2
Explanation:
The balanced equation of this chemical reaction is
2 MnO2 + 4 KOH + O2 → 2 KMnO4 + 2 KOH + H2
2 moles of MnO2 produces 2 moles of KMnO4
That means 1 moles of KMnO4 will be produced from 1 moles of MnO2
Hence, 1.5 moles of KMnO4 will be produced from 1.5 moles of MnO2
The moles of manganese oxide were used in the reaction is 1.5 moles.
What is stoichiometry?Stoichiometry of any reaction tells about the relative amount of species present before and after the chemical reaction.
Given chemical reaction is:
2MnO₂ + 4KOH + O₂ → 2KMnO₄ + 2KOH + H₂
From the stoichiometry of the reaction, it is clear that same moles of manganese oxide and potassium permanganate is involved in the reaction.
2 moles of KMnO₄ = produce by 2 moles of MnO₂
1.5 moles of KMnO₄ = produce by 2/2×1.5=1.5 moles of MnO₂
Hence, required moles are 1.5 moles.
To know more about moles, visit the below link:
https://brainly.com/question/24631381
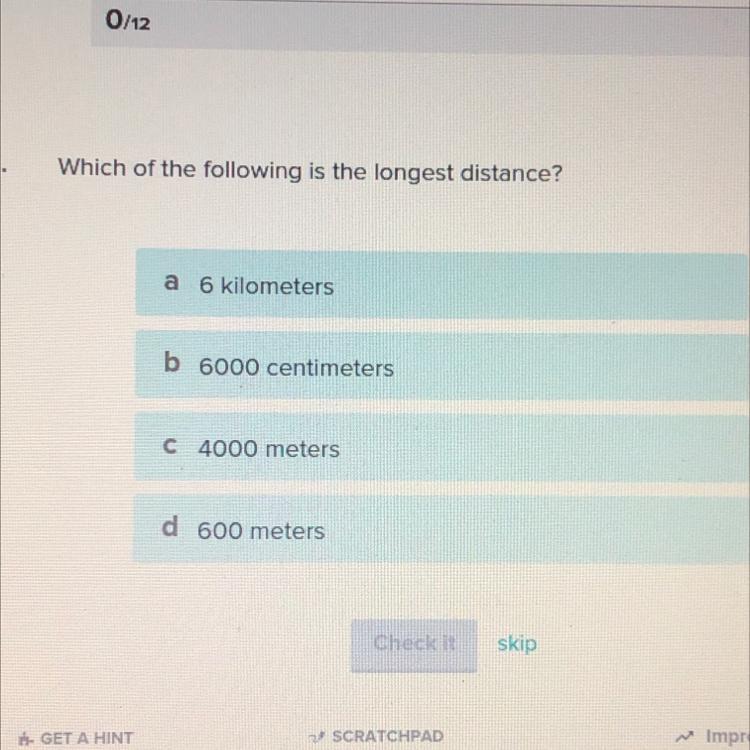
Help. I’ll give brainliest.
Answers
Answer:
obviously it is 6 km cause that's the longest length
Answer:
the answer is A
Explanation:
Its A because 6km is the longest and 1Km = 1000 meters
The boiling point of water is 100ºC. The boiling point of acetone is 56ºC. Which statement about distilling a mixture of acetone and water is correct?(1 point)
Acetone remains in the original container.
Acetone remains in the original container.
Water will vaporize from the mixture before acetone.
Water will vaporize from the mixture before acetone.
Water is collected as it leaves the mixture.
Water is collected as it leaves the mixture.
Acetone will vaporize from the mixture before water.
PLSSS HELPPP!!!!

Answers
Answer:
I think Acetone will be captured and cooled.
Explanation:
Distillation is a method of separation based on the difference in boiling point of two liquids.
The liquid that has a lower boiling point is first separated from the mixture. It vaporizes, cooled and collected before the liquid that has a higher boiling point.
In this case, acetone is captured and cooled before water since it has a boiling point of 56ºC and water has a boiling point of 100ºC.
Answer:
Acetone will vaporize from the mixture before water.
Explanation:
This is for the people in the future!
Mixtures Quick Check!
(The comment was accidental, I took the test and this was the answer)
Which statement is true of any reversible reaction?
A. It can proceed in either direction
B. It can occur only between gases
C. It has only one product
D. It has more than one reactant

Answers
Answer:
A. It can proceed in either direction
Explanation:
A P E X
Reactions can be reversible and irreversible. The reversible reactions can proceed in either direction. Thus, option A is correct.
What are reversible reactions?Reversible reactions can convert the reactants and products into each other and hence, move in both the left and right directions. These types of reactions never get completed and attain equilibrium.
The reactants react to produce the products, and the products re-react to form the reactants of the reaction mixture. The melting of the wax can be a reversible reaction.
Therefore, option A. reversible reactions can occur in both directions is correct.
Learn more about the reversible reaction here:
https://brainly.com/question/23711430
#SPJ2
The enthalpy of combustion for octane (C8H18(l)), a key component of gasoline, is -5,074 kJ/mol. This value is the Delta. Hrxn for which of the following reactions? 2C8H18(l) 25O2(g) Right arrow. 16CO2(g) 18H2O(g) C8H18(l) 12. 5O2(g) Right arrow. 8CO2(g) 9H2O(g) 16CO2(g) 18H2O(g) Right arrow. 2C8H18(l) 25O2(g) 8CO2(g) 9H2O(g) Right arrow. C8H18(l) 12. 5O2(g).
Answers
Combustion can be defined as the reaction of a compound with oxygen. The enthalpy of combustion of octane is \(\Delta H_{\rm rxn}\) for \(\rm C_8H_{18}\;+\;25\;O_2\;\rightarrow 8\;CO_2\;+\;9\;H_2O\).
What is the enthalpy of reaction?The enthalpy of reaction is the amount of heat energy absorbed or lost by the molecules in the chemical reaction.
The enthalpy of combustion is the amount of heat energy released by the compound in the reaction with oxygen.
The reaction in which heat is liberated with the reaction of a compound with oxygen has an enthalpy of combustion, equivalent to the enthalpy of reaction.
The combustion of octane can be given as:
\(\rm C_8H_{18}\;+\;25\;O_2\;\rightarrow 8\;CO_2\;+\;9\;H_2O\)
Thus, the reaction has combustion energy equivalent to the enthalpy of the reaction is \(\rm C_8H_{18}\;+\;25\;O_2\;\rightarrow 8\;CO_2\;+\;9\;H_2O\). Thus, option B is correct.
Learn more about enthalpy of reaction, here:
https://brainly.com/question/1657608
what happens to the volume of an ideal gas if its pressure is halved and its kelvin temperature is doubled, assuming the moles of gas does not change? what happens to the volume of an ideal gas if its pressure is halved and its kelvin temperature is doubled, assuming the moles of gas does not change? the volume remains constant the volume decreases by a factor of 4 the volume quadruples the volume doubles
Answers
The volume of an ideal gas if its pressure is halved and its kelvin temperature is doubled than the volume quadruples.
The ideal gas equation is given as :
P V = n RT
from the question , is given that :
P2 = P1 / 2
T2 = 2 T1
then from the ideal gas equation we get :
P1 V1 / T1 = P2 V2 / T2
P1 V1 / T1 = ( P1 / 2 × V2 ) 2 T1
V2 = 4V1
Thus, The volume of an ideal gas if its pressure is halved and its kelvin temperature is doubled than the volume quadruples.
To learn more about ideal gas here
https://brainly.com/question/28583355
#SPJ4
What is the distance traversed by the particle between 0 seconds and 6 seconds
Answers
The distance travelled by the particle between 0 seconds and 6 seconds is 12 m
What is velocity?Velocity is simply defined as the rate of change of displacement with time. Mathematically, it can be expressed as:
Velocity = displacement / time
From the question given above, the following data were obtained:
Time = 6 sVelocity = 2 m/sDisplacement =?Velocity = displacement / time
The displacement of the object between 0 and 6 s is calculated as;
2 = displacement / 6
Cross multiply
Displacement = 2 × 6
Displacement = 12 m
Learn more about velocity here:
brainly.com/question/3411682
#SPJ1

A pharmaceutical manufacturer conducted a crossover study with an anticonvul- sant drug (DPH) used in the management of grand mal and psychomotor seizures. A single dose of DPH was given to a subject, and the plasma level of the drug was measured 12 hours after the drug was administered. The four treatments were (A) 100 mg generic DPH product in solution, (B) 100 mg manufacturer DPH in cap- sule, (C) 100 mg generic DPH product in capsule, and (D) 300 mg manufacturer DPH in capsule.
Answers
Based on the information provided, the pharmaceutical manufacturer conducted a crossover study to compare the effectiveness of different formulations of an anticonvulsant drug (DPH) used to manage grand mal and psychomotor seizures.
The four treatments involved are:
(A) 100 mg generic DPH product in solution
(B) 100 mg manufacturer DPH in capsule
(C) 100 mg generic DPH product in capsule
(D) 300 mg manufacturer DPH in capsule
In this study, a single dose of DPH was given to a subject, and the plasma level of the drug was measured 12 hours after the drug was administered. The goal of this study is to compare the bioavailability of these different treatments to determine the most effective dosage and formulation of the anticonvulsant drug.
To know more about anticonvulsant, visit:
https://brainly.com/question/28209828
#SPJ11
Which of the following describes an interaction between the musculoskeletal system and the respiratory system?
The air sacs absorb more oxygen during vigorous exercise.
The air sacs are used due to signals from the brain.
The blood delivers glucose and oxygen to cells.
The blood carries waste materials to the kidneys.
Answers
The statement that describes an interaction between the musculoskeletal system and the respiratory system is as follows:
The air sacs absorb more oxygen during vigorous exercise.Thus, the correct option is A.
What is the Musculoskeletal system?The musculoskeletal system may be defined as a system of numerous organs that assists in the maintenance of the body's overall structure and framework. This system provides humans the capability to move through the utilization of their muscles and skeletal systems.
The primary function of the respiratory system is to perform the gaseous exchange and make the blood rich in oxygen and exhale carbon dioxide.
The Musculoskeletal system helps in the active movement of the body and requires oxygenated blood to accomplish the process of muscle contraction and body movements.
And thus this system requires enhanced oxygen supply during the time of vigorous physical activity that assists the movement.
Therefore, the correct option for this question is A.
To learn more about the Musculoskeletal system, refer to the link:
https://brainly.com/question/22699765
#SPJ1
What is always correct about the molecular ion, M+, in a mass spectrum of a compound? a) The M+ ion peak has the smallest m/z ratio in the mass spectrum. b) The m/z ratio of the M+ ion peak gives the relative molecular mass of the molecule. c) The M+ ion is the most stable fragment formed during electron bombardment. d) The M+ ion peak has the greatest intensity in the mass spectrum.
Answers
The m/z ratio of the M+ ion peak gives the relative molecular mass of the molecule.
correct option is B
The molecular ion, M+, in a mass spectrum is the precursor ion of the molecule before fragmentation.
The m/z ratio of the M+ ion peak in a mass spectrum gives the relative molecular mass of the molecule.
The m/z ratio is the ratio of the mass of the ion to its charge, and for the M+ ion, this value is equal to the molecular mass of the compound.
By measuring the m/z ratio of the M+ ion peak, it is possible to determine the relative molecular mass of the molecule, which is a crucial piece of information for identifying and characterizing a compound.
to learn more about molecular ion refer here
https://brainly.com/question/20815761#
#SPJ11
a chemical company makes a silver by reacting silver nitrate would see the company needs to make 800 g of pure silver for a client they have 300 g of zinc and 600 g of silver nitrate will they be able to make enough silver to fill the order
Answers
Answer
Explanation
Given that:
The mass of pure silver needed = 800 g
Mass of zinc = 300 g
Mass of silver nitrate = 600 g
What to find:
Will the mass of zinc and silver nitrate be able to make 800 g of pure silver.
Step-by-step solution:
Step 1: Write the balanced equation for the reaction.
Zn + 2AgNO₃ → 2Ag + Zn(NO₃)₂
Step 2: Determine the moles of the reactants.
Using the mole formula, the moles of the reactants will be:
\(\begin{gathered} Moles\text{ }of\text{ }Zn=\frac{Mass}{Molar\text{ }mass}=\frac{300g}{65.38g\text{/}mol}=4.5886\text{ }mol \\ \\ Moles\text{ }of\text{ }AgNO_3=\frac{600g}{169.87g\text{/}mol}=3.5321\text{ }mol \end{gathered}\)Step 3: Determine the moles of pure silver produced.
Using the mole ratio of Zn to AgNO₃ in the equation and the moles in step 2, we
A 500-ml glass beaker is filled to the brim with ethyl alcohol at a temperature of 5. 00ºc. how much will overflow when its temperature reaches 22. 0ºc?
Answers
The volume of ethyl alcohol overflow will be 9.265mL.
To calculate the volume of ethyl alcohol overflow, we will use
∆V=V(á∆T)
Where,
∆V=volume expand
á=volumetric expansion Coefficients =0.00109/°C
V=initial volume =500ml
T'=final temp. =22°C
T=initial temp=5°C
Now,
∆V=500×(0.00109/°C) ×(22-5)
=500×(0.00109) (17)
=9.265ml
Thus, we find that the volume of ethyl alcohol overflow will be 9.265 mL.
learn more about ethyl alcohol:
https://brainly.com/question/11237501
#SPJ4
What is an algal bloom?
A. a growth of new algae
B. a type of floating algae
C. an overgrowth of algae
D.a type of flowering algae
Answers
Answer:
C im pretty sure. hope this helps
Put the following elements in order from largest to smallest atomic radius
Br, Fe, Ga, Ca, Cu
Answers
Answer:
Ca-197
Ga-135
Cu-128
Fe-126
Br-114
Can you do anything to reduce the amount of error that might occur in estimating the area covered by an object?
Answers
Reducing error in area estimation is an ongoing process. Continuous practice and refinement will improve your estimation skills over the long term. By applying these techniques and taking your time, you can minimize errors and enhance the accuracy of your estimates.
To reduce the amount of error that might occur in estimating the area covered by an object, there are several steps you can take: 1. Break down the task: Instead of trying to estimate the area of the entire object at once, divide it into smaller, more manageable sections.
2. Use a grid or ruler: By overlaying a grid or using a ruler, you can create a more systematic approach to estimating the area.
3. Approximate with familiar shapes: If the object is irregularly shaped, try to approximate it using familiar shapes such as rectangles, triangles, or circles.
4. Utilize technology: Take advantage of digital tools or software that can assist with measuring and estimating areas.
5. Verify and iterate: After estimating the area, compare it to a known or measured value, if available.
Remember, reducing error in area estimation is an ongoing process. Continuous practice and refinement will improve your estimation skills over the long term. By applying these techniques and taking your time, you can minimize errors and enhance the accuracy of your estimates.
To know more about error visit:
brainly.com/question/30384710
#SPJ11
a que llamamos niveles y subniveles de energia?
Answers
Answer:
agua
Explanation:
por que estamos en la planeta
Philosophy ultimately seeks to discover ultimate truth
True or false
Answers
Answer:
True
Explanation:
Phislosophy does not accept something even though it is a khown fact unless it can proves it's true .
Descartes sais that in philosophy we must doubt the facts we khow , we must start from the beginning to reach the truth
is scent considered a physical property
Answers
Answer:
yep!
Explanation:
How do I add up all the energy to get the total energy emitted?

Answers
Answer:
To calculate the amount of heat released in a chemical reaction, use the equation Q = mc ΔT, where Q is the heat energy transferred (in joules), m is the mass of the liquid being heated (in kilograms), c is the specific heat capacity of the liquid (joule per kilogram degrees Celsius).
Explanation:
Select the alcohol oxygens that will be oxidized to a ketone functional group when the following molecule is treated with chromic acid.
Answers
This problem is asking for the ketone that will be formed when an specific alcohol is oxidized with chromic acid. Although there is no attached file or molecule, you should select the secondary alcohol.
Oxidation of alcoholsIn organic chemistry, oxidation reactions such as those producing aldehydes and carboxylic acids from primary alcohols and those producing ketones from secondary alcohols are based on either the increase of oxygen atoms or the decrease of hydrogen atoms in the molecule.
However, primary carbons are those having one bond with another carbon atom, and they lead to the formation of aldehydes and carboxylic acids as shown below:
\(R-CH_2-OH\rightarrow R-HC=O\rightarrow R-C(=O)-OH\)
On the other hand, secondary alcohols are those having the OH at a carbon with two bonds with two different carbon atoms and lead to formation of ketones upon the oxidation with chromic acid:
\(R_1-CH(OH)-R_2\rightarrow R_1-C(=O)-R_2\)
In such a way, you must select the secondary alcohol for your answer, regardless not included in the question you shared.
Learn more about ketones: https://brainly.com/question/4439718
evidence of a chemical change includes?
Answers
Answer:
Change of color, change in temperature, change of energy or loss heat, odor etc....
The average atomic mass or atomic weight found on the periodic table is
Answers
Answer:
the average mass of all isotopes of that element. It is located below the element symbol.
The average atomic mass or atomic weight on the periodic table is the average atomic mass of all the isotopes of that particular element.
What are isotopes?
Isotopes are defined as substances having same number of protons but different number of neutrons.Number of protons is characteristic for determining position of elements in the periodic table.
Since,all isotopes have the same number of protons and hence have same position.They have similar chemical properties as they have same number of electrons.
They find applications in the field of nuclear medicine and oil and gas research . There are 2 types of isotopes : stable and unstable
Unstable isotopes are radioactive and are called as radioisotopes.Some of these isotopes are man -made and hence also called as artificial isotopes.Every element has an isotope which is either man-made or natural .
Many properties of isotopes depend on mass which is measured in atomic mass unit. The difference in actual mass and mass number is called mass defect.
Learn more about isotopes,here:
https://brainly.com/question/27475737
#SPJ2
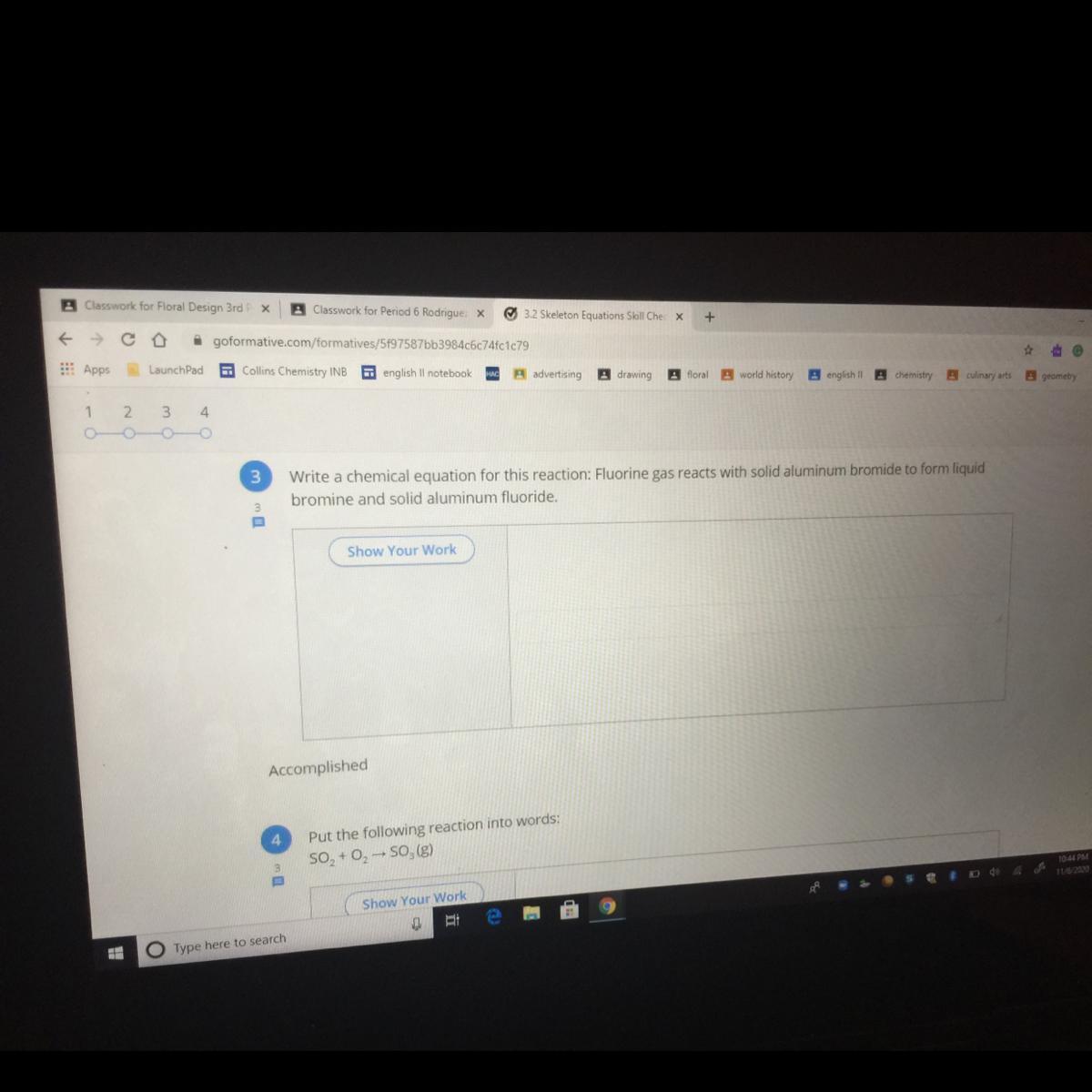
i really need these answered!!
Answers
Explanation:
3)
Equation -
3F₂ (g) + 2AlBr₃ (s) → 3Br₂ (l) + 2AlF₃ ↓
4)
When sulphur dioxide gas reacts with oxygen then it forms sulphur trioxide gas.
Equation -
2SO₂ (g) + O₂ (g) → 2SO₃ (g)
Which phrase best describes the role carbon plays in the structure of compounds present in living things? no role no role a minimal role a minimal role a somewhat important role a somewhat important role a fundamental role
Answers
Carbon plays a fundamental role in the structure of organic compounds.
What role does carbon play in compound structure?Organic compounds are primarily composed of carbon. Carbon, like many other elements, can form stable bonds with itself. Organic compounds are classified into four types: carbohydrates, lipids, proteins, and nucleic acids.Without carbon, there would be no life on Earth. This is due, in part, to carbon's ability to easily form bonds with other atoms, which allows for greater flexibility in the form and function of biomolecules such as DNA and RNA, which are essential for the defining characteristics of life: expansion and replication.Carbon helps to regulate the Earth's temperature, allows all life to exist, is a key component of the food we eat, and is a major source of energy for our global economy.Carbon's ability to form stable bonds with many elements, including itself, is the reason for this. This property enables carbon to form a wide range of extremely large and complex molecules.To learn more about carbon play in compound structure refer to
https://brainly.com/question/24419453
#SPJ4