shortly after ad 1000, biruni, an arabic physician, composed a pharmacology book with the first written description of
Answers
Shortly after AD 1000, Biruni, an Arabic physician, composed a pharmacology book with the first written description of various drugs and their uses.
This book provided detailed information on the effects and side effects of different medicines, as well as instructions on how to prepare and administer them. Biruni's work laid the foundation for modern pharmacology and greatly contributed to the development of medicine as a science.
Biruni, an Arabic physician, composed a pharmacology book shortly after AD 1000. This book contained the first written description of various medicinal substances, their properties, and their uses in treating diseases. By incorporating detailed information on pharmacology, Biruni's work significantly contributed to the understanding and advancement of medical knowledge during that time period.
Learn more about pharmacology here:
https://brainly.com/question/30308277
#SPJ11
It is believed that the pharmacology book composed by Biruni shortly after AD 1000 contained the first written description of the process of distillation.
This technique involves heating a liquid mixture to vaporize certain compounds, which are then condensed back into a liquid form and collected separately.
Biruni's description of distillation is considered significant because it paved the way for the development of many important chemical processes, such as the production of essential oils, perfumes, and alcoholic beverages.
Additionally, distillation has played a key role in the development of modern chemistry and is still widely used today in a variety of industries, including pharmaceuticals, petroleum refining, and food and beverage production.
For more question on pharmacology click on
https://brainly.com/question/368331
#SPJ11
Related Questions
Protein A has a binding site for ligand X with a dissociation constant, Kd, of 3.0 x 10-7 M. Protein B has a binding site for ligand X with a K of 4.0 x 10 M. Calculate the K, for each protein.
Answers
The breakdown constant, Kd, of the binding site onto protein A for ligand X is 3.0 x 10-7 M. A ligand X binding site on protein B does indeed have a K value 4.0 x 10 M. K has a ratio of 0.133.
Describe protein.Protein, which is located in practically every cell, muscle, other body part, encompassing muscle, osteoporosis, skin, and hair, makes up the human body. It helps to produce enzymes, which power countless phase changes, and haemoglobin, which carries oxygen in the blood.
Protein A kd = 3.0 x 10⁻⁷ M
Protein B kd = 4.0 x 10⁻⁸ M
ka = ?
Protein A ka = 1/kd
= 1/ 3.0 x 10⁻⁷
ka = 0.33 * 10⁷m⁻
Protein B ka = 1/kd
= 1/ 4* 10⁻⁸
= 0.25 * 10⁸
ka = 2.5 * 10⁷ m⁻
ratio of k = 4.0 x 10⁻⁸/3.0 x 10⁻⁷
= 0.133
To know more about protein visit:
https://brainly.com/question/29776206
#SPJ4
A student describes the motion of particles in each of the four different states of matter. State 1: Particles that are charged move freely State 2: Particles move freely at high speed State 3: Particles are locked in place State 4: Particles slide past one another Which state describes plasma? Group of answer choices State 1 State 2 State 3 State 4
Answers
Answer:
state 1
Explanation:
Particles that are charged move freely State describes the state describing plasma. Stage 1 is correct.
What is plasma?Plasma is an ionized gas in which the ions that are positively charged can move freely and electrons can also move freely with low pressure and high temperature in the gaseous state of the matter.
In plasma, there are only charged particles present that can only move freely with no pressure it is an ionized gas because the formation of gas is done after only the ionization process and these ionized particles can only move.
Therefore, Stage 1 is correct. Particles that are charged move freely State describes the state describing plasma.
Learn more about plasma , here:
https://brainly.com/question/18207038
#SPJ2
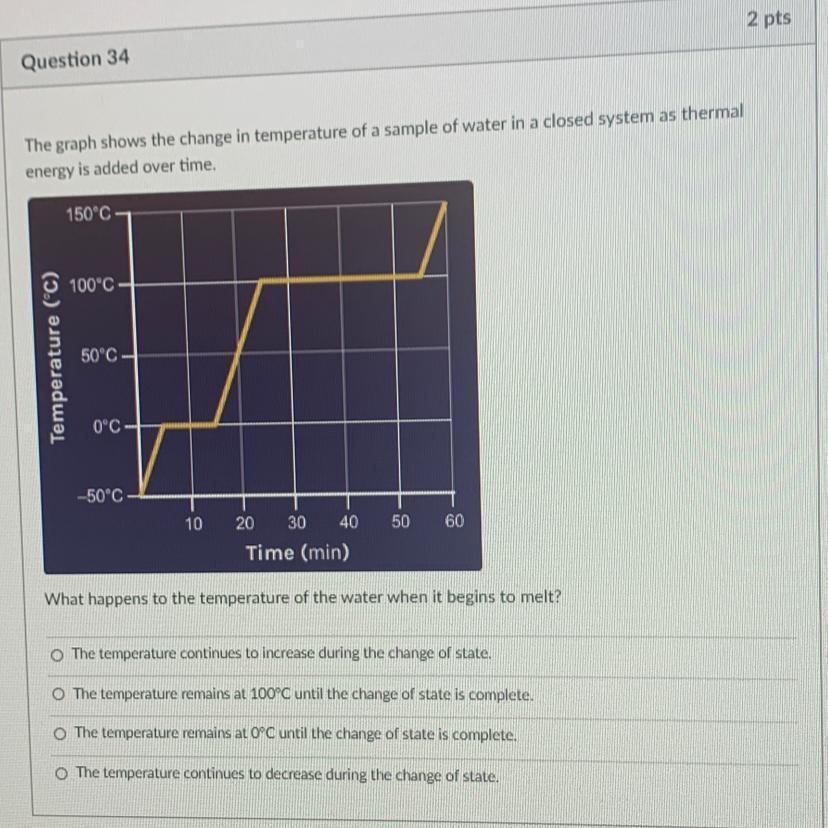
Temperature(°C)
The graph shows the change in temperature of a sample
of water in a closed system as thermal energy is added
over time.
150°C
100°C
50°C
Answers
The graph shows that as the status changes, the water's temperature rises steadily. As a result, choice A is the right response to this query.
What will happen if the ice melts?The amount of seawater would often rise as the ice melted. This is due to the tendency of solid ice to turn liquid over time. And as a result, the density of the seawater may now increase, raising the level.
When the temperature falls to 0°C, it is observed. It might stay the same for a while, but as time goes on, it usually gets bigger.
When the temperature hits 100°C, the same theory is evident. Therefore, it cannot be argued that the temperature stays at 0°C or 100°C prior to state transitions.
Thus, the correct option is A.
For more details regarding Temperature and change of state, visit:
https://brainly.com/question/27988898
#SPJ1
Your question seems incomplete, the missing image is:
An unknown amount of gas has a volume of 12 L and a pressure of 5.6 atm. If the temperature is 205K, how many moles of gas are present
Answers
The number of moles of the gas present is 4 moles.
To calculate the numbers of moles of the gas present, we use the formula below.
Formula:
PV = nRT............. Equation 1Where:
P = PressureV = Volumen = Number of moles of the gasR = molar gas constantT = Temperature.Make n the subject of the equation
n = PV/RT............ Equation 2From the question,
Given:
P = 5.6 atmV = 12 LT = 205 KR = 0.0821 L.atm.K⁻¹.mol⁻¹Substitute these values into equation 2
n = (5.6×12)/(0.0821×205)n = 67.2/16.8305n = 3.99n ≈ 4 molesHence, the number of moles of the gas present is 4 moles.
Learn more about number of moles here: https://brainly.com/question/21911991
What does the energy hill represent on an energy diagram?
A. The potential energy gained by the products when a reaction
happens
B. The potential energy the reactants have stored in molecular bonds
O C. The additional potential energy the reactants must gain in order to
react
D. The final amount of potential energy of the products of the
reaction
Answers
B.The potential energy the reactants have stored in molecular bonds
25 pts
3. Describe the subatomic structure of the nucleus, including the structure of each nucleon. Draw a picture.
Describe the forces that hold the nucleus together and draw them on your diagram.
Explain how beta emission works
Answers
Answer:
2 circles one proton and one nucleon.draw quarks within each. strong nuclear force within protons between quarks and residual strong force between proton and nucleon (up,up,down in proton)
Explanation:
What is the [H+] if the pH of a solution is 2.0?
[ ? ] × 10¹²] X [H+] =
Answers
The concentration of hydrogen ions in the solution of pH 2.0 is 0.01 mol/L
What is the [H+] if the pH of a solution is 2.0?The pH of a solution is a measure of the concentration of hydrogen ions, H+ in the solution.
The relationship between pH and the concentration of hydrogen ions is given by the equation:
pH = -log[H+]
Rearranging the equation gives:
[H+] = 10^(-pH)
Substituting pH = 2.0 into this equation gives:
[H+] = 10^(-2.0)
[H+] = 0.01 mol/L
Learn more about pH at: https://brainly.com/question/12609985
#SPJ1
Calculate the volume of a liquid with a density of 5.45/mL and a mass of 65 g
Answers
Answer:
11.93 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \\\)
From the question we have
\(volume = \frac{65}{5.45} \\ = 11.926605\)
We have the final answer as
11.93 mLHope this helps you
A(g) + B(g) = C(g) + D(g)
18. For which of the following equilibrium constants will reactions of the general type shown above
give the greatest yield of products C+D when equilibrium is attained ?
(A) K= 1 x 10^-15
(B) K= 1 x 10-²
(C) K= 1 x 10²
(D) K= 1 x 10^50
Answers
Comparing the given equilibrium constants, we find that K values increase from (A) to (D): 1 x 10^-15 < 1 x 10^-2 < 1 x 10^2 < 1 x 10^50.
Since a higher K value signifies a greater yield of products, the greatest yield of products C and D will be obtained when the equilibrium constant is the highest. the answer is (D) K = 1 x 10^50.
option d
The equilibrium constant (K) is a measure of the extent of a chemical reaction at equilibrium. It is defined as the ratio of the concentrations (or partial pressures) of the products to the concentrations (or partial pressures) of the reactants, each raised to the power of their respective stoichiometric coefficients.
In the given reaction A(g) + B(g) = C(g) + D(g), a higher equilibrium constant (K) indicates a greater yield of products C and D at equilibrium. This means that the reaction is more favorable in the forward direction, leading to a higher concentration of C and D compared to A and B.
A high equilibrium constant indicates that the reaction strongly favors the formation of products C and D and will shift towards the products' side at equilibrium. In this case, the forward reaction is highly favored, resulting in a significant yield of products C and D.
option d
for more such questions on equilibrium
https://brainly.com/question/19340344
#SPJ8
How many moles are present in 5.67x10^25 atoms of Carbon (C)?
Answers
Answer:
94.155 Moles
Explanation:
Since you are given atoms, to get to moles you need to use the formula I used below. Pretty simple, just plug and chug. Work can be found below.
Hope this helps! :^)
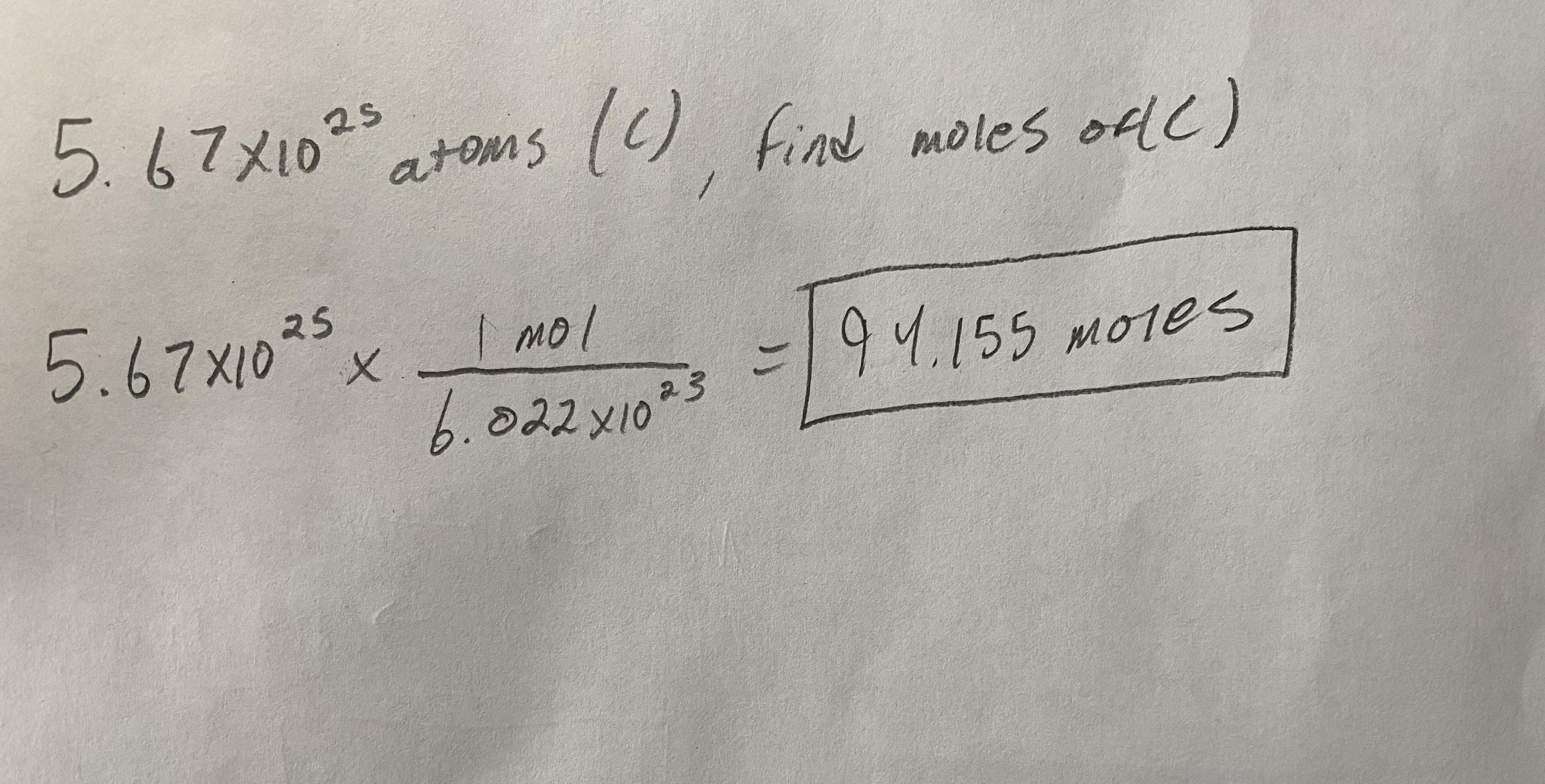
94. 125 moles are present in 5.67x10^25 atoms of Carbon (C).
What is mole?
The mole is a unit of measurement that is comparable to well-known units like pair, dozen, bulk, etc. It offers a precise count of atoms or molecules in a large sample of materials.
The amount of substance that has the same number of discrete entities (atoms, molecules, ions, etc.) as the number of atoms in a sample of pure 12C weighing exactly 12 g is known as a mole.
The word "mole" has a "big mass" or "bulk" sense in Latin, which is congruent with its use as the name for this unit. The mole establishes a connection between bulk mass, which is a simple macroscopic attribute to measure, and the number of atoms, molecules, and other fundamental units, which is a crucial fundamental property.
Therefore, 94. 125 moles are present in 5.67x10^25 atoms of Carbon
(C).
To learn more about mole, refer to the link:
https://brainly.com/question/26416088
#SPJ2
Which statement below is true about vaporization?
A. Vaporization occurs when liquid changes to a solid.
B. Vaporization occurs when particles lose energy and become sold.
C. Vaporization occurs when there is an decrease in heat.
D. Vaporization occurs when liquid particles gain energy and "fly away" into a gas.
Answers
Answer:
Explanation:
D. Vaporization occurs with liquid particles gain energy and "fly away" into a gas.
Think of when you're boiling something on the stove.
Someone please answer this in like 40 minutes
Before it’s due. Explain the answer too

Answers
Answer:
kkkwkisa
Explanation:
wulaoaiq8qiIq8qiaik
You removed a Coke can from the refrigerator. When you come back a few minutes later, you realize the can has begun to "sweat".
In other words, moisture forms on the outside of the can. Select ALL of the statements that describe the formation of condensation
on the can.
)
A)
Water vapor in the air gains energy, causing it to form condensation on the
can
B)
As water molecules in the air come in contact with the cold can, they will
slow down.
C)
As water molecules in the warm air collide with the cold can, they will lose
energy and become colder.
D)
Vapor molecules in the air are attracted to water molecules on the can
causing them to bind to the can.
E)
Condensation on the can comes from water vapor in the air cooling and
slowing and becoming liquid on the outside of the can
Answers
Answer:
B
Explanation:
The statements that describe the formation of condensation on the can are
B) As water molecules in the air come in contact with the cold can, they will slow down.
C) As water molecules in the warm air collide with the cold can, they will lose energy and become colder.
E) Condensation on the can comes from water vapor in the air cooling and slowing and becoming liquid on the outside of the can.
What is condensation?Condensation is the process of converting water vapor into liquid. The process happens when the temperature lowers and the vapor in the air condenses and converts into water molecules.
Here, the condensation occurs near the cold can, the surrounding air condenses, lowered the energy and converts it into water molecules.
Thus, the correct options are B, C, and E.
To learn more about condensation, refer to the below link:
https://brainly.com/question/15563071
#SPJ6
during chemiosmosis in aerobic respiration, protons are pumped __________.
Answers
Electrons are passed through a series of redox reactions, and each transfer causes protons to be pumped across the membrane. This creates a concentration gradient, which is used to power ATP synthesis through the process of chemiosmosis.
During chemiosmosis in aerobic respiration, protons are pumped across the inner mitochondrial membrane from the matrix to the intermembrane space.
Aerobic respiration is a process of producing energy that involves the complete breakdown of glucose in the presence of oxygen. It is a crucial metabolic pathway that is present in all higher organisms, including humans.Chemiosmosis is the process in which a transmembrane electrochemical gradient drives ATP synthesis. It is an important part of cellular respiration and oxidative phosphorylation.
During the process of oxidative phosphorylation, protons are pumped across the inner mitochondrial membrane, which creates a proton gradient that powers the synthesis of ATP. In aerobic respiration, the electron transport chain (ETC) is the primary mechanism that generates the proton gradient.
Electrons are passed through a series of redox reactions, and each transfer causes protons to be pumped across the membrane. This creates a concentration gradient, which is used to power ATP synthesis through the process of chemiosmosis.
to know more about Aerobic respiration visit :
https://brainly.com/question/11874459
#SP11
How many atoms of nitrogen are in 1.5 moles of aspartame?
Answers
Answer:
459.11 g.
Explanation:
hope this helps
Why is water considered to be polar?
Answers
Answer: Water is a polar molecule since it has an unequal sharing of electrons. Water is chemically written as H2O meaning it consists of hydrogen and oxygen atoms. Hydrogen is number one in the Periodic Table whereas oxygen is number 14. As a result, the configuration of oxygen is 2.8.4 while that of hydrogen is 1. When two hydrogen atoms combine with one oxygen atom, two out of the four electrons in oxygen form the strong bond in water. The resulting effect is that there is an unequal sharing of electrons since two electrons remain unused. The hydrogen end becomes partially positive while the oxygen end is partially negative. Furthermore, the oxygen atom has a stronger attractive force hence drawing more atoms to it. Subsequently, there arises a charge imbalance within the molecule. Besides water, hydrogen fluoride is also a polar molecule.
Explanation: I hope this answerd your question have a good day :)
please can someone help me

Answers
The both can be separated by adding water to the mixture.
How can you separate diamonds from sugar?Diamonds and sugar are two very different substances, and it is relatively easy to separate them from each other using physical and chemical methods.
One common method to separate diamonds from sugar is by using their different physical properties. Diamonds have a much higher density than sugar, so if you mix a sample of both substances in water, the diamonds will sink to the bottom while the sugar will float on top and dissolve in the water. This is known as gravity separation.
Learn more about diamond:https://brainly.com/question/29775108
#SPJ1
The pH of a solution is 8. Some hydrochloric acid is added to the solution. Suggest the pH of the solution after mixing. pH=.......??
Answers
Answer:
Probably around 6 because the ph of hydrochloric acid is 3
Explanation:
calculate the mass of 25,000 molecules of nitrogen gas. (1 mole = 6.02 × 1023 molecules) group of answer choices 7.00 × 105 g 5.81 × 10−19 g 5.38 × 1026 g 1.16 × 10−18 g
Answers
The mass of the 25,000 molecules of Nitrogen gas is found to be 0.0000017 g (1.7 x 10⁻⁶ g).
We must use the molar mass of nitrogen and the Avogadro's number to get the mass of 25,000 molecules of nitrogen gas.
Nitrogen (N₂) has a molar mass of 28 g/mol.
The number of particles in one mole of any substance determined by Avogadro is 6.02 x 10²³.
One nitrogen gas molecule's mass can be determined as follows:
Nitrogen's (N₂) molecular weight is 28 g/mol.
Nitrogen gas molecules total 25,000.
Nitrogen gas moles equal (25,000 molecules) / (6.02 x 10²³ molecules/mol)
(Num. of moles) x = mass of nitrogen gas (molar mass)
[(25,000 molecules) / (6.02 x 10²³ molecules/mol)] is a formula for the mass of nitrogen gas. x (28 g/mol)
Nitrogen gas mass = 0.00000117 g
Therefore, 25,000 nitrogen gas molecules have a mass of about 0.00000117 g.
To know more about Avogadro number, visit,
https://brainly.com/question/859564
#SPJ4
One main difference between a Prokaryotic cell and an Eukaryotic cells is __________.
Question 3 options:
Prokaryotic cells have many organelles
The Eukaryotic Cell has a nucleus
The Prokaryotic cells have a cell wall
The Eukaryotic cell is the only cell with DNA
Answers
Answer:
The Eukaryotic cell has a nucleus
Explanation:
Prokaryotic cells lack a nucleus and membrane-bound structures. Eukaryotic cells have a nucleus and membrane-bound structures called organelles.
Given the unbalanced equation: what is the coefficient of al2(so4)3 when the equation is completely balanced using the smallest whole-number coefficients?.
Answers
The coefficient of Al₂(SO₄)₃ when balance is 1. Al₂(SO₄)₃. Since Al₂(SO₄)₃ is a balance structure, the coefficient does not need to be add.
How to balance substance in a reaction?Let's say we have unbalance reaction CaSO₄ + AlCl₃ → Al₂(SO₄)₃ + CaCl₂. To make it balance, follow this steps:
Count all atom in the reactant and the product. Balance the number on thee reactant and productGive the coefficient for every substance in the reactionTry to re-check to make sure that you did not a mistake.The result of the example will be:
3CaSO₄ + 2AlCl₃ → Al₂(SO₄)₃ + 3CaCl₂
Learn more about chemistry reaction here
https://brainly.com/question/11231920
#SPJ4
How many elements are in H2O
Answers
Answer: bale 2 elements sya ang mga yon ay hydrogen at oxygen.
H2O is a molecule made of H for hydrogen and O for oxygen. There are 12 of these molecules. The smaller number is the number of atoms of the element to the left of it.
MARK ME AS A BRAINLIEST PLEASE
H₂O, which represents a molecule of water, consists of three atoms: two hydrogen atoms (H) and one oxygen atom (O). Therefore, H₂O contains a total of three elements: hydrogen and oxygen.
What is H₂O?H₂O represents a molecule of water. The "H" stands for hydrogen, and the "O" stands for oxygen.
Hydrogen (H) is a chemical element with the atomic number 1. It is the lightest and most abundant element in the universe. Hydrogen atoms consist of a single proton in the nucleus and one electron in orbit around the nucleus.
Oxygen (O) is a chemical element with the atomic number 8. It is a highly reactive and abundant element, making up a significant portion of the Earth's atmosphere and the compounds found on Earth. Oxygen atoms consist of eight protons in the nucleus and eight electrons in orbit around the nucleus.
Learn more about elements at
https://brainly.com/question/20096027
#SPJ6
Choose all of the examples that show a chemical change.
A) A woodpecker digs in the bark.
B) A dead tree rots on the ground.
C) A forest fire burns a lot of trees.
D) A farmer is cutting logs with an axe.
E) A tree drops rainwater from its leaves.
Answers
Answer:
B and C
Explanation:
Answer:
B) A dead tree rots on the ground.
C) A forest fire burns a lot of trees.
Explanation:
It cannot return the the state it was in before or have the same composition it had before.
PLEASE HELP !!!
According to the
graph, what happens
to the concentration
of A over time?
(n) uonenu ว
Reaction: 2A A,
Time (sec)
A. It decreases and then levels out.
B. It decreases consistently.
C. It increases and then levels out.
D. It increases consistently.

Answers
According to the graph, the concentration of A decreases with time before leveling out. Option A.
Concentration of a reactant in a reversible reactionThe reaction shown is that of a reversible reaction in which A is on the reactant's side and A2 is on the product's side.
At the beginning of the reaction, the concentration of A decreases as a result of forming A2. In other words, the concentration of A2 increases just as that of A decreases.
With time, the reaction reaches an equilibrium during which the rate of formation of A equals the rate of formation of A2. At this point, the concentration of A levels off.
In summary, the concentration of A first decreases before leveling off.
More on reversible reactions can be found here: https://brainly.com/question/31950205
#SPJ1
If 15 g of CzHo reacts with 60.0 g of Oz, how many moles of water (H2O)
will be produced? (Hint: use what you know about limiting reactant) *
2 C2H6 + 7 O2 + 4 CO2 + 6 H2O
O 1.5 mol H20
O
1.6 mol H20
O
1.0 mol H20
O
1.1 mol H20
Answers
Answer:
1.5 mol H2O
Explanation:
Limiting reactant was C2H6 - 0.5 moles
Fomula became .5C2H6 + 1.75O2 = 1CO2 + 1.5H2O
3. A wave has a wavelength of 10 m and a speed of 340 m/s. What is the frequency of the
wave?
Answers
Answer:
We have the wavelength which is 10,speed/velocity=340 and frequency=?.
The formula is velocity=frequency × wavelength.
340=f × 10.
340=10f.
frequency=34hertz.
Answer:
34hertz
Explanation:
We have the wavelength which is 10,speed/velocity=340 and frequency=?.
The formula is velocity=frequency × wavelength.
340=f × 10.
340=10f.
frequency=34hertz.
Which statement best describes a property of a compound? A compound is made up of a single atom. B. A compound is made up of many atoms that are all the same type. C. A compound can be separated into two or more elements through physical processes. D. A compound can be separated into two or more elements through a chemical reaction.
Answers
Answer:
D. A compound can be separated into two or more elements through a chemical reaction.
Explanation:
By method of elimination, we are going t obtain the correct option.
A. A compound is made up of a single atom.
This is wrong because, a compound can contain more than one atom. An example is H2O
B. A compound is made up of many atoms that are all the same type.
This is wrong because a compound can contain atoms of different elements. An example is H2O. It contains atoms of hydrogen and oxygen.
C. A compound can be separated into two or more elements through physical processes.
This is wrong. H2O being a compound cannot be separated by ordinary physical means.
D. A compound can be separated into two or more elements through a chemical reaction.
This is the correct option.
How many moles are in 1.2 x 10^24 formula units of Li₂SO4? (round your answer to the nearest tenths place)
Answers
In 1.2 x \(10^{24}\) formula units of \(Li_{2} (SO)_{4}\), there are roughly 1.993 moles of
\(Li_{2} (SO)_{4}\).
How many moles of \(Li_{2} (SO)_{4}\) are contained in 1.2 x \(10^{24}\) formula units?Using Avogadro's number, or 6.022 x \(10^{23}\) molecules/mol, we can calculate the number of moles of Li2SO4 in 1.2 x \(10^{24}\)formula units.
First, we need to figure out how many moles of \(Li_{2} (SO)_{4}\) are needed to equal 1.2 x \(10^{24}\) formula units:
Formula units equal 6.022 x \(10^{23}\) per mole of \(Li_{2}(SO)_{4}\).
As a result, there are: 1.2 x \(10^{24}\) moles of \(Li_{2}(SO)_{4}\) in the formula units.
1.993 moles are equal to 1.2 x \(10^{24}\) formula units / 6.022 x \(10^{23}\) formula units/mol.
Hence, 1.2 × \(10^{24}\) formula units of \(Li_{2} (SO)_{4}\) contain about 1.993 moles.
To learn more about moles visit:
brainly.com/question/26416088
#SPJ9
how many valence electrons are there in an argon atom?
Answers
Answer:
The element argon is in column 8A and is a noble gas. Nobles gases are completely stable because their outershell is filled with all eight electrons. Argon has eight valence electrons.
The element X forms the chloride XCl4 ( 4 being a sub script of Cl ) containing 75% Cl by mass. What is element X?
Answers
The element X forms the chloride XCl₄ ( 4 being a sub script of Cl ) containing 75% Cl by mass. The X element in the compound is titanium.
The molecular weight of chlorine is 35.5 g/mol.
Four chlorine atoms, or molecules, make up the chemical XCl₄and account for 0.75 of its total weight.
All four chlorine atoms weigh a total of 4×35 = 140 g/mol
The compound's total weight of all chlorine atoms is now 140/0.75.
The compound's overall weight is now equal to 186.66≈ 187
The mass of element X is equal to 187 - 140 = 47 g/mol.
If we match any element from the periodic table, we can discover that the molecular weight of titanium is almost equivalent to 47.
TiCl₄ is the substance, and its molecular weight is 140 g/mol.
Learn more about Molecular weight here
brainly.com/question/26388921
#SPJ4